
|
83 BISMUTH Bi (German: bisemutum)
Bismuth is a brittle metal having a silvery lustre with a pink tinge which is stable in air and water, but burns with a blue flame forming yellow fumes of the oxide when heated. It is an extremely rare and toxic element but the high insolubility of most of its compounds renders it relatively safe. Bismuth is strongly diamagnetic, repelling a magnetic field, and also has a low thermal neutron cross sectional area, making it suitable as a liquid metal coolant for nuclear reactors. A high absorption of gamma rays makes it a useful filter for these whilst transmitting neutrons. Bismuth is magnetoresistive, increasing in resistance with increasing magnetic fields, and coiled in a 'bismuth spiral' is used in magnetic flux meters. Bismuth has the lowest thermal conductivity of any metal except mercury, and a high electrical resistivity. Bismuth is one of the few elements (gallium is another) to expand on solidification (by 3.2%). As one metal in a thermocouple, it has the highest negativity known. In the Hall effect, a slab of conducting material with a current flowing through it generates a voltage across its width when a magnetic field is applied across the sample. The electrons are deflected towards the sides of the conductor by the magnetic field, generating the transverse voltage. The magnitude of this effect varies for differing materials, in bismuth it is very large.
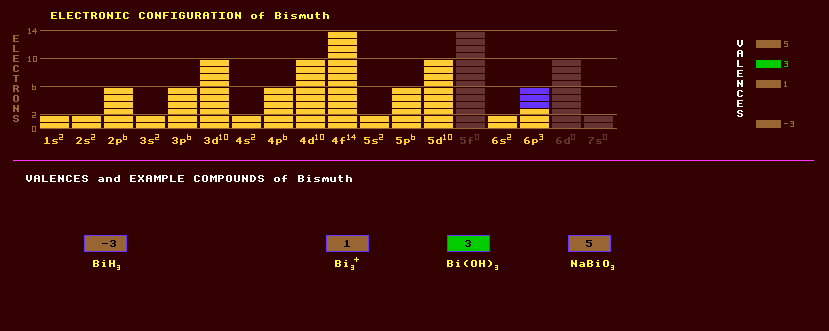
Bismuth is a component of some of the lowest melting point alloys: Woods metal (4Bi,2Pb,Sn,Cd) melts at 71ºC, Rose's metal (2Bi,Pb,Sn) melts at 93.75ºC and Lipowitz alloy (15Bi,8Pb,4Sn,3Cd) which melts at 60-65ºC, which are used as fusible alloys e.g. in as fusible plugs in water sprinklers. Bismuth exhibits the valences -3, 1, 3 and 5. The known oxides are Bi2O3 and the unstable Bi2O5. Halides include BiF3, BiF5 and KBiF6. A volatile unstable hydride, bismuthine, BiH3, can be made. Bismuth trichloride, BiCl3, reacts with water forming bismuth oxychloride, sometimes used as a pigment under the name 'pearl white' in cosmetics. Bismuth oxide, Bi2O3, forms when bismuth is heated in air, and has marked basic properties rather than acidic ones. Barium titanate, BaTiO3, has a massive dielectric constant making it suitable for use in hi-k ceramic capacitors and is both piezoelectric and ferroelectric with a Curie temperature of 135 Celsius, see lithium niobate under niobium. Bismuth telluride is semiconductor with poor thermal conductivity, which makes it an ideal semiconductor for exploiting the thermoelectric effect exhibited by a p-n junction, which manifests itself in two complementary manners, the Peltier and Seebeck effects: When a junction between a heavily p-doped region and a heavily n-doped region is formed in bismuth telluride and a current is passed through the junction, one side of the junction becomes hot whilst the other side becomes cold, this is the basis of a Peltier cooler device, used for cooling other chips to reduce electronic noise, especially in sensitive infra-red imaging devices. Passing a current the other way will reverse the effect. The Seebeck effect is the opposite of the Peltier effect; a differential temperature across the junction generates a voltage across the junction, useful for generating small amounts of electricity for example in heart pacemakers, or for heat flux sensors. The semiconductors bismuth selenide and bismuth antimonide also make excellent thermoelectric coolers, but neither these nor bismuth telluride will work below 200 Kelvin. A good thermoelectric material should have a high electrical resistivity whilst having a low thermal conductivity and a high Seebeck coefficient (coupling between heat flow and electric current). The alloy with manganese, BiMn, can make very strong permanent magnets. Ores of bismuth include bismite, the monoclinic phase of bismuth trioxide alpha-Bi2O3. Sillenite, a secondary mineral, is the cubic phase of the trioxide, polymorphic with bismite. Bismuthinite, or bismuth glance, Bi2S3, the best ore of bismuth, occurs in hydrothermal veins as shapeless lead-grey masses commonly associated with chalcopyrite, and has a yellowish tarnish. The corresponding telluride, tellurobismuthinite, Bi2Te3, is trigonal and belongs to the tetradymite group. Much rarer higher tellurides exist: pilsenite, Bi4Te3 and hedleyite, Bi7Te3. A tetragonal carbonate, bismutite, (BiO2)CO3, is also mined but the main source of bismuth is a by-product of lead and copper smelters. Bismuth ochre is a group name for undetermined oxides and carbonates occurring as earthy masses. Schapbachite or mataldite, AgBiS2, is rare. Native bismuth occurs in lamellar masses of a rose pink colour tarnished with an irridescent film, and is the main source of the metal. Bismuth is the last element in the periodic table to have any stable isotopes and consists entirely of just one stable isotope, bismuth-209. A trace of the alpha decaying radioactive isotope bismuth-210, which has a halflife of just 5 days, is found in uranium ores, a secular equilibrium product of uranium-238 decay. Altogether, a total of 28 radioactive isotopes of bismuth are known, from the alpha emitting Bi-187 to the beta decaying Bi-215. Bismuth-109, once thought to be non-radioactive, is not totally stable, and will decay by alpha decay, although it's half-life is extremely long at 1.9 × 10-19 years. Because its radioactivity is so very weak, it has only recently become possible, in 2003, to measure the halflife of such feebly radioactive isotopes.
Claim to fame: Bismuth has the highest diamagnetic susceptibility (-280
|
![]()