
|
82 LEAD Pb (Latin: Plumbum)
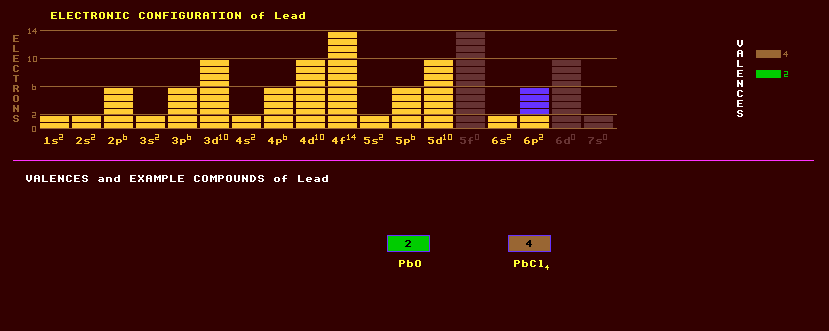
Lead is a shiny bluish-white dense metal which is soft, ductile and malleable but often has a dull grey appearance due to the formation of a protective basic carbonate patina. It crystallizes in the cubic system, is a poor electrical conductor, and subject to mechanical creep. Lead was the first element found to exhibit a property called superconductivity, where electrical resistivity drops to zero at and below a superconducting critical temperature, which for lead is 7.19 Kelvin. Lead is used as the anode in rechargeable (secondary) lead acid batteries (accumulators) , and was also once commonly used for pipes in lead plumbing. Due to its 'dead' nature, it is used as a sound barrier and vibration damper. Also used as waterproof flashings around chimney stacks; as a neutron shield around nuclear reactors and as a shield for X-rays; and for lead shot. Alloyed with tin, and sometimes a little copper, it is used in solder and in pewter drinking tankards, in type metal, in leaded window lattices, and in white metal bearings, sometimes hardened with a little arsenic or antimony. Lead exhibits just two valences, +2 and +4. Many compounds of lead are highly coloured and used as paint pigments: the mixed oxide Pb3O4, red lead, white lead, PbHCO3 and PbSO4; the yellow or red PbO; chrome yellow, PbCrO4; orange lead, a mixture of PbO2 and Pb3O4; the oxychloride, Turners yellow, PbCl2.4PbO; and basic lead chromate or chrome red, PbCrO4.PbO. Lead oxide is used to dope glass to produce 'lead-crystal' glass, and the higher refractive index flint glass for achromatic lenses. Lead acetate, Pb(COO.CH3)4·H2O, or sugar of lead, was once used as a sweetener for drinks by the Romans, but slowly poisoned them. Tetraethyl Lead, Pb(CH5)4, is used as an anti-knock additive in leaded petrol. Lead azide, Pb(N3)2, is used as a detonator of high explosives. Known oxides: lead monoxide, PbO, forms two crystalline forms (massicot, a yellow rhombic powder and a red tetragonal form, litharge); lead dioxide, PbO2; the reddish yellow lead sesquioxide, Pb2O3 (or plumbous plumbate, PbPbO3); red lead, Pb3O4 (or plumbous orthoplumbate Pb2(PbO4); and Pb5O8. Lead sulphide has useful thermoelectric properties up to 700 Celsius, and is used as the Seebeck effect element in thermoelectric generators. In the human body, lead (and its compounds) is a cumulative poison producing the characteristic neurological symptoms of heavy-metal poisoning and is a common environmental pollutant. Like all heavy metal poisons, it's effects are insidious. Environmental concern has almost eliminated use of lead in paints and petrol. Lead is commonly found in association with silver amongst quartz rock, where it occurs as the ore, galena, lead sulphide, PbS, which is black and shiny like coal. Red lead ore, crocoite, PbCrO4, lead chromate, is a comparatively rare mineral occurring as bright red crystals associated with galena. Other less common ores are the sulphate, anglesite, PbSO4; the white carbonate, cerussite, PbCO3, known to miners as lead white, is a product of the weathering of galena; the red oxide, minium, Pb3O4; the yellow-green chlorophosphate, pyromorphite, Pb5(PO4)3Cl and the related mineral containing vanadium instead of phosphorus called vanadinite, Pb5(VO4)3Cl, which is more useful as an ore of vanadium; the yellow or lime green chloroarsenite, mimetite, Pb5(AsO4)3Cl; the yellow to orange campylite, Pb5[(As,P)O4]3Cl, a mixture of mimetite and pyromorphite; phosgenite, Pb[ClCO3]; the azure blue linarite, PbCu(OH)2SO4; the yellow rare molybdate, wulfenite, PbMoO4; the colourless sulphocarbonate, leadhillite, 3PbCO3.PbSO4; the colourless and basic sulphate, lanarkite, PbO.PbSO4; and the pale blue, plumbogummite, PbAl3(PO4)2(OH)5.H2O Caledonite, Cu2Pb5(SO4)CO3(OH)6, is deep blue. There are several lead antimony sulphides: monoclinic jamesonite, Pb4FeSb6S14; monoclinic boulangerite, Pb5Sb4S11; plumosite, Pb5Sb6S14; meneghinite, Pb13Sb7S23; monoclinic robinsonite, Pb4Sb6S13; monoclinic semseyite, Pb9Sb8S21; monoclinic plagiorite, Pb5Sb8S17; and hexagonal zinkenite, Pb6Sb4S11, all associated with lead and antimony ores, some very rare. Jordanite, Pb14(As,Sb)6S23, monoclinic, is rare and forms a series with geochronite. Lead occurs rarely in elemental form. Natural lead is a mixture of four stable isotopes, the un-common Pb-204, Pb-206, Pb-207 and the most abundant Pb-208. A trace of two beta decaying radioactive isotopes is also found on earth, lead-210 with halflife 23 years, and lead-214 with halflife 27 minutes. Lead is the end product of three radioactive decay chains, Pb-206 the uranium series; Pb-207 the thorium series; and Pb-208 the actinium series. All 29 other known isotopes are radioactive. Claim to fame:
|
![]()