
|
81 THALLIUM Tl (Greek: thallos = green twig)
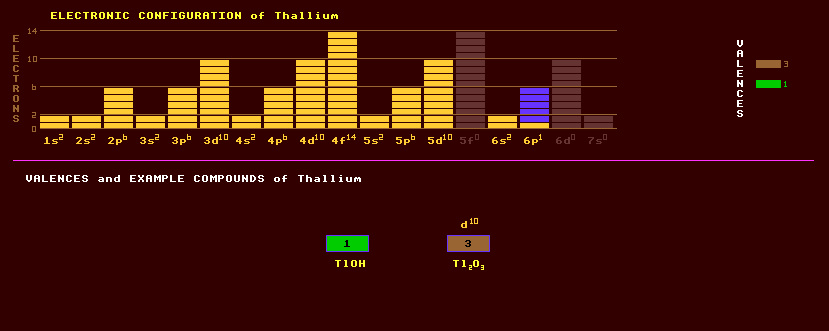
Thallium is a toxic, silvery-grey metal which is soft and malleable like lead. When first exposed to air thallium has a metallic lustre but soon develops a bluish-grey tinge resembling lead. Further exposure to moist air or steam results in the formation of thallium hydroxide, TlOH. Thallium is attacked by acids, and rapidly dissolves in nitric acid. The element was named after the beautiful green spectral line which first identified the element. Thallium exhibits two valences, 1 and 3. The oxides, Tl2O, and Tl2O3 are known. It is little used because of its toxicity. Skin contact with the metal is dangerous, and adequate ventilation should be provided when melting the metal. Thallium sulphate is widely used as a rodenticide and ant killer; it is tasteless and odourless giving no warning of its presence. Thallium sulphide is used in photocells because its electrical resistivity changes with exposure to infra-red light. Thallium bromide-iodide crystals have been used in infra-red detectors. Thallium has ben used, with sulphur or selenium and arsenic to produce low melting point glass which melts between 125 Celsius and 150 Celsius. Thallium oxide is used to produce glass with a high refractive index. Thallium is rare, and found dispersed in potash, feldspar and pollucite, (Cs,Na)AlSi2O6.nH2O. It is obtained commercially as a by product of zinc and lead smelting. Thallium also occurs in crooksite, lorandite (thallium arsenic sulphide), hutchinsonite, and pyrite from which it is extracted. The radioactive isotope, thallium-201 which has a half-life of 73 hours, is injected internally for heart-scans. Thallium patients have, after up to six days after their scan, set off sensitive radiation alarms at airports etc. As these radiation detectors become more sensitive, more such radiation alarms will be triggered by more patients a longer period after their radioactive injection. Natural thallium is a mixture of two stable isotopes, 70% thallium-205 and 30% thallium-203. A trace of thallium-208, a beta decaying isotope, occurs naturally despite having a very short halflife of 3 minutes. Several thallium isotopes are members of the uranium, actinium, neptunium and thorium radioactive series. Thallium isotopes are used in scintillation crystals. Altogether, 29 radioactive isotopes of thallium are known ranging from the alpha decaying thallium-179 to the beta decaying thallium-210. Claim to fame:
|
![]()