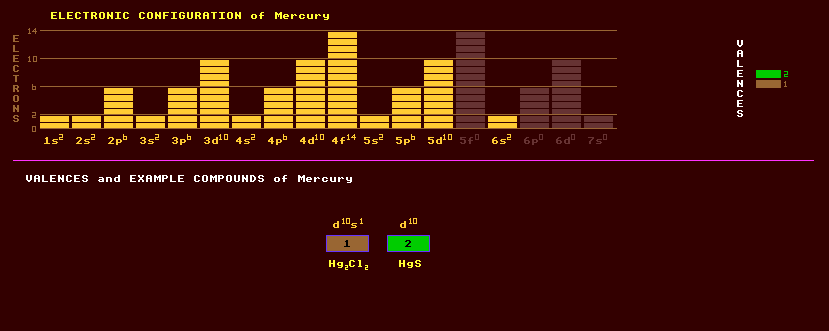

|
80 MERCURY Hg (planet & L: Hydrargyrum = liquid silver)
Mercury is a dense shiny white metal, also known as quicksilver, and the only metal to be liquid at room temperature, with the exception of gallium on hot days. It is used as the temperature sensing element in mercury thermometers. Mercury solidifies at -39ºC and boils at 350ºC. Because of its high density, it is used in barometers to measure atmospheric pressure and as a floating bearing for the revolving optics of lighthouses, and because it conducts electricity, in mercury-wetted relays and tilt switches. New uses are in upward pointing astronomical telescopes where a liquid mercury bath is spun to form a parabolic mirror. It is used in mercury batteries to provide a stable voltage of 1.35 volts. In an electrolytic cell, it is used to measure electrical charge, or elapsed time and power-on hours in electronic apparatus. Mercury will dissolve most other metals, forming what is called an amalgam. Tin or gold amalgams find use as a tooth filling in dentistry. Mercury is also used to extract gold and silver, but in doing so, pollutes the rivers of many third world countries. Mercurial soap containing calomel, mercurous chloride, Hg2Cl2, is used as a germicidal soap. Mercuric chloride, HgCl2, corrosive sublimate and a virulent poison, is an important salt. Mercury fulminate, Hg(ONC)2, is used as an initiatory explosive in detonators and toy caps. Methyl mercury is used as a grain pesticide. Mercury vapour lamps are used to illuminate roads and as a source of ultra-violet light, which in fluorescent lamps, excites a phosphor coating around the tube. The electrical discharge within the lamps cause mercury to combine with any noble gases present to form HgNe, HgAr, HgKr, and HgXe, held together by Van der Waal forces. Mercury arc rectifiers are used in electrical power machinery. Mercury has an extremely low vapour pressure, and because the vapours are exceedingly poisonous, makes its use hazardous. Air at room temperature saturated with mercury vapour contains 100 times the toxic limit of 0.05mg per cubic metre. Broken thermometers slowly kill! Mercury, and especially its organic compounds, are highly poisonous producing the characteristic neurological symptoms of heavy metal poisoning which include disturbed coordination, tremors, deafness, tunnel vision, all caused by severe brain damage. Mercury is an insidious cumulative poison that is readily absorbed through the skin. In the environment, mercury can be converted by organisms in mould and in marine organisms to the much more toxic dimethyl mercury, (CH3)2Hg, which is easily absorbed by the body passing into the brain. Marine animals are particularly at risc; tuna especially has been found with enhanced concentrations of mercury. The expression 'mad-as-a-hatter' came about through cases of mercury poisoning by use of mercury in the hat making process. Several serious cases of mass poisoning have resulted Worldwide by the use of mercury compounds as anti-fungal treatment for grain, by the use of mercury for gold extraction, and by eating mercury contaminated fish. The biggest source of environmental mercury pollution is coal-fired power stations, where mercury contained in the coal is released and vaporised up the chimney. At present, 4000 million tons of coal are burnt annually worldwide (2004). No way has yet been found to trap the mercury before it is emitted, it defies all tried chemical traps and scrubbers. In a glass beaker liquid mercury displays the unusual property of emitting tiny flashes of light at the glass/metal interface when stirred - thought to be caused by electrostatic charges discharging at the moment of slip in the stick/slip friction exhibited by mercury and glass in relative motion. Red mercury may be a hoax. Russian scientists claim to have produced an explosive cherry red gel like substance of mercury and antimony oxides, Hg2Sb2O7, by irradiating them in nuclear reactors for 20 days. The red mercury is said to contain 100-1000 times more chemical energy than the most powerful high explosive known, others doubt this claim saying it would need bonds between inner electrons likely to be unstable to accomplish this feat. But could not hollow atoms be involved, where the inner electrons are absent but outer ones still present? Principal ores are cinnabar, mercury sulphide, HgS, occurring as bright red crystals used as the pigment vermilion; and calomel, Hg2Cl2, a white or grey mass. Schwazite, or hermesite, (CuHg)3SbS4, is a grey to black granular mass associated with mercury ores. Native mercury is sometimes found in small quantities associated with cinnabar. Moschellandsbergite, Ag2Hg3, is a silvery white amalgam with silver occurring as rhombic dodecahedrons associated with calomel. Mercury exists as seven stable isotopes, comprising 30% mercury-202, 23% mercury-200, 17% mercury-199, 13% mercury-201, 10% mercury-198, 7% mercury-204 and just 0.2% mercury-196. A further 26 radioactive isotopes are known, ranging from the alpha decaying mercury-175 to the beta decaying mercury-207. Claim to fame: Mercury is the only metal to be liquid at room temperature. Superconductivity was first discovered in a ring of metallic mercury when it levitated on a magnetic field as it was cooled down with liquid helium to less than 4 degrees above absolute zero. Mercury has the lowest Latent Heat of Atomization (61KJ/mol) of any element.
|
![]()