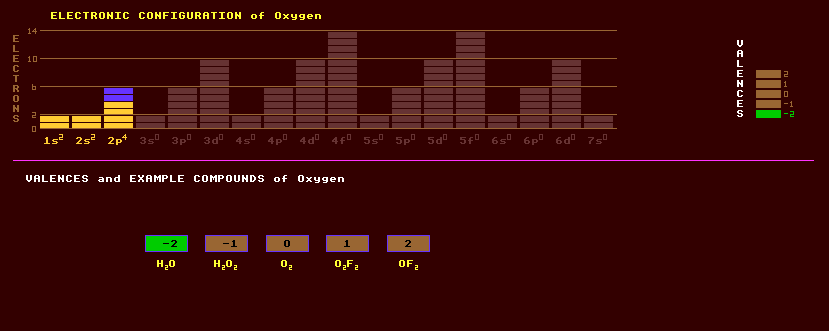

|
8 OXYGEN O (Greek: oxy genes = acid forming)
Oxygen is an odourless, colourless diatomic gas normally present in the atmosphere at a concentration of 20.9%, although it has not always been so in the geological past, when a reducing atmosphere rather than an oxidising atmosphere was present. On Earth, 89% of the oxygen is contained in water, and 50% in rocks, from where it is thought our atmospheric oxygen originated. Oxygen is now exhaled into the air by plants during photosynthesis, the source of atmospheric oxygen. Animals consume oxygen during respiration, exhaling carbon dioxide. Oxygen, a highly reactive chemical, is thus by these means maintained in a steady state concentration in the atmosphere which is far from chemical equilibrium. The residence time for oxygen in the atmosphere is about 3000 years, that is, on average, each molecule of oxygen is re-cycled from/into the atmosphere every 3000 years. (By comparison, the residence times for atmospheric nitrogen is 20 million years, whilst that for carbon dioxide is only 2 years. Hence atmospheric concentrations of CO2 can vary year by year, but that of N2 is almost static). Oxygen is an extremely reactive gas, and if it were present in higher concentrations, trees would spontaneously ignite. It is thought that forest fires control the present concentrations of oxygen in the atmosphere, but concentrations have reached 35% in the geological past. An allotrope of oxygen, ozone, O3, is formed in the upper atmosphere by the ultraviolet photo-dissociation of oxygen into the highly reactive atomic oxygen, O, and its subsequent recombination with diatomic oxygen, O2. Because ozone absorbs harmful ultraviolet light from the sun, it protects life on Earth. The recent destruction of stratospheric ozone at the Earths' poles each spring is of serious concern and is indirectly caused by chloro-fluorocarbons, CFCs, refrigerants and propellants from from refrigerators and aerosol cans respectively, which have a long atmospheric residence times because of their chemical inertness. The CFCs permeate the atmosphere up to the stratosphere, where they are decomposed by UV light from the sun into the highly reactive atomic chlorine, Cl. The chlorine, catalyses the destruction of ozone on the surface of ice crystals in freezing ice clouds in the presence of UV light. This only occurs at the intensely cold temperatures of -80ºC coupled with sunshine, which occurs only at the poles in spring. The destruction of ozone can reach 80% over a very large area. Ozone at ground level is caused by photochemical reactions with atmospheric pollutants from automobiles, which are mainly oxides of nitrogen. It can also be formed wherever a silent electrical discharge is occurring, such as in photocopiers, or some so-called electrical 'air fresheners'. Ozone is pungent in extremely small concentrations, reminiscent of chlorine, and extremely poisonous. If you can smell it, it is causing harm. Ozone is a powerful oxidant and bleach. With hydrogen, oxygen can form inert water, H2O, and hydrogen peroxide, H2O2, another oxidant, used as a household bleach in 10% solutions, or as an oxidant propellant in rocket engines powered by hydrazine fuel, N2H4. Oxygen forms many compounds, and is also responsible for the tarnishing, or, together with the action of water, corrosion (in the case of iron, rusting) of many metals when exposed to the atmosphere. When a substance is said to be burning, it is reacting with oxygen releasing heat energy in an uncontrolled way. Most elements react with oxygen to form oxides. Many metal oxides are white and highly refractory, but some highly coloured and used as pigments in prehistoric cave paintings and in modern paints. Oxygen, meaning acid producer, is compounded in many acids, like sulphuric acid, H2SO4, but not in all, being notable absent from hydrochloric acid, HCl, and hydrofluoric acid, HF. Oxygen can be produced in the laboratory either by electrolysing water or by heating potassium chlorate, KClO3 with manganese dioxide, MnO2. Obtained commercially by the fractional distillation of liquefied air. Three stable isotopes of oxygen can exist, O-16, O-17 and O-18. Oxygen-16 is by far the most common, with an isotopic abundance of 99.8%, followed by O-18 at 0.018%. There is just a small proportion of O-17. The ratio of thee isotopes in air trapped deep down in Arctic pack ice can give clues to the Earths climatological and atmospheric past. In addition 10 radioactive isotopes are known from O-12 to O-24. Claim to fame: Oxygen has the highest lithospheric, crustal and human abundance of any element.
|
![]()