
|
79 GOLD Au (Latin: Aurum)
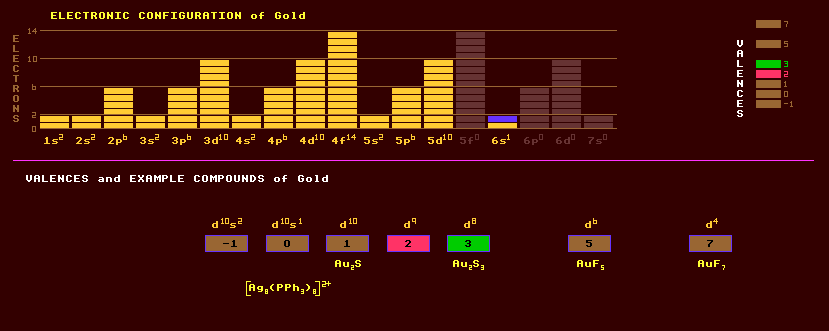
Gold is an expensive heavy, soft, malleable and ductile precious metal of the transition series and is pale yellow by reflected light, and can be beaten into very thin gold leaf which appears green by transmitted light. The most malleable element. Finely divided, it may be black, ruby or purple. It is an excellent conductor of heat and electricity. Most of the Worlds gold is uselessly locked away in vaults as national currency reserves. Gold is used in coinage and jewellery, where it is alloyed with varying amounts of copper and silver to confer greater hardness. The carat is used as a measure of the purity of gold, 24 carat being pure gold. White gold is an alloy with nickel, but as used in dentistry the alloy contains platinum or palladium. Gold leaf is used for the decoration (gilding) of books and buildings. Gold leaf electrometers are used to measure static electrical voltages and ionising radiation. Gold film glass is glass with an embedded meandering stripe of gold which when electrically heated will de-mist the glass. Gold is used to colour some glass, and also as a heat-reflective layer on some windows to reduce heat-loss from buildings. Gold plated aluminium foil is used to cover satellites to reflect solar radiation and prevent the satellite becoming too hot. Gold plating connectors are used in electronic circuitry for good electrical contact being resistant to corrosion. Gold is insoluble in all acids except aqua regia, a mixture of one part nitric and three parts hydrochloric acids, when it forms chlorauric acid, HAuCl4. Gold toning of photographs is done with chlorauric acid. Gold can adopt a valency of +1 or +3. Gold is not essential for life, but a gold compound, disodium aurothiomalate, is administered in the treatment of arthritis. Gold is primarily associated with high temperature hydrothermal quartz veins in extrusive rocks. Gold is often found in association with pyrites, or Fools Gold, FeS2, (iron sulphide) with which it is sometimes confused; and stibnite (antimony sulphide). Gold will form liquid complexes with hydrogen sulphide, H2S, under the extreme conditions found deep underground, and it is thought that when this hot liquid comes into contact with iron bearing rocks, the sulphur reacts with the iron forming iron sulphide whilst the gold is deposited. Also, heat-resistant gold-loving bacteria living on hot underwater volcanic plumes in deep oceanic trenches accumulate the gold from the H2S/Au complexes, and this is thought responsible for some deposits of gold. Ores include the gold silver tellurides sylvanite, AgAuTe4 and petzite, Ag3AuTe2, plus selenides of gold. Gold occurs in native form as nuggets or grains usually in river beds where it has been deposited (placer deposits) by erosion of rocks higher up, but is more usually found minutely disseminated throughout some rocks. South African gold, found in a bed of pebbles up to 6 kilometres deep (formed as a river bed sank) is minable down to only 4km. When found as gold plates, tetragonally shaped crystals protrude like pyramids from the surface. Gold also occurs as gold amalgam containing 60% mercury in Columbia and California. Also frequently found as a natural alloy with silver (electrum), and less often with palladium (porpezite), and rhodium (rhodite). Sea water contains about 0.03mg of gold per cubic metre, mainly as the gold chloride complex [AuCl2]-, but with no known economic means of extraction. Gold is recovered from its ores with potassium cyanide and mercury amalgam and smelting, and by electrolysis, with great potential for pollution of the environment. A cheaper alternative solution for dissolving gold, a mixture of iodine, tetraethylammonium iodide and acetonitrile, was discovered in 1997. This liquid becomes saturated with gold at its boiling point of 82ºC, and precipitates it out at 20ºC, and could become an important method for extracting gold. Gold has only one stable isotope, Au-197, and its melting point of 1064.43ºC is a useful standard for calibrating temperature scales. A radioactive isotope of gold, Au-198, a beta emitter with a half-life of 2.7 days, is used in the treatment of cancer.
|
![]()