
|
78 PLATINUM Pt (Spanish: platina = silver)
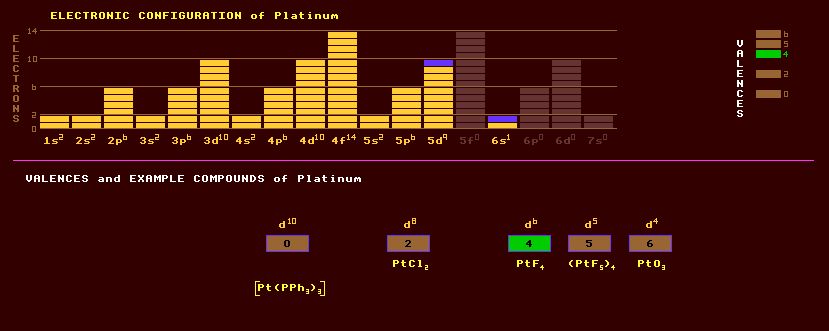
Platinum is a heavy, lustrous, silvery-white, soft, malleable and ductile metal unaffected by oxygen and water, dissolving only in aqua regia and fused alkalis, halogens, cyanides and sulphur. It resists attack from the highly corrosive hydrogen fluoride gas, HF. Platinum crystallizes in the cubic system and is the founder and most abundant and important member of the platinum group of rare metals comprising osmium, iridium, palladium, rhodium and ruthenium. Platinums' resistance to corrosion and highly reproducible temperature coefficient of electrical resistance makes it eminently suitable as the sensing element in platinum resistance thermometry for use over an extended temperature range. It is also used as one half of a thermocouple element. Platinum is used in jewellery and scientific apparatus. Also used for electrical contacts subject to high temperatures and for electrodes subject to chemical attack. Also used to coat missile cones and jet engine nozzles which must perform reliably at high temperatures. Platinum will absorb large volumes of hydrogen at room temperature, but gives it off when heated to red heat. Platinum is used extensively as a catalyst: Asbestos permeated with finely divided platinum, platinized asbestos, is used for cracking petroleum products. Platinum black is platinum precipitated from a solution of platinum tetrachloride, PtCl4, and used as a catalyst. Platinum is used as a catalyst in automobile catalytic converters, combatting pollution, but emitting noxious fumes of hydrogen sulphide when not hot enough! Fine platinum wire glows hot in town gas, used in gas lighters. It converts methyl alcohol vapour to formaldehyde, the basis of hand warmers. A mixture of the gases hydrogen and oxygen will explode in its presence. Platinum has a thermal expansion coefficient almost identical to that of soda-lime-silica glass and is therefore used to seal electrodes in some bulbs. Platinite contains no platinum and is an alloy of about 55% iron and 44% nickel with a trace of carbon and has the same expansion coefficient as platinum, which it is used to replace in some light bulbs and measuring standards, like copies of the standard metre. A 76.7% platinum 23.3% cobalt alloy makes an extremely powerful magnet with an energy almost twice that of Alnico-V. The standard metre in Paris consists of an alloy of 10% iridium and 90% platinum. Platinum exhibits the valences of 0, 2, 4, 5 and 6. Known oxides: PtO, PtO2, and PtO3. The five valent platinum is represented by (PtF5)4. Platinum readily forms complexes used as drugs and reagents. Platinic hydroxide, Pt(OH)4 dissolves in acids to form platinic salts and in bases to form platinates. Platinic oxide, or platinum dioxide, PtO2 is a dark grey powder obtained when platinic hydroxide is heated. Platinous hydroxide, Pt(OH)2 is soluble in acids such as HCl forming platinous salts. Platinous oxide, PtO, is formed when platinous hydroxide is heated. Platinum dissolves in aqua regia forming the important compound chloroplatinic acid, H2PtCl6. Platinum has no known role in life, but cis-platin is used as a potent anti-cancer treatment which works by combining with DNA thus preventing cell division; it is therefore very toxic. Unusual amongst the noble metals, a mineral ore of platinum is found, speryllite, platinum arsenide, PtAs2, a minor ore. Platinum is also found in the native, free state, but usually alloyed with iridium, rhodium, palladium or osmium, or occasionally with tellurium. Commercially, platinum is extracted as a by product of copper and nickel refining. Natural platinum is slightly radioactive and occurs as a mixture of four stable isotopes and two slightly radioactive isotope. The stable isotopes comprise 34% platinum-195, 33% platinum-194, 25% platinum-196, and just 7% platinum-198. The radioactive isotopes comprise 0.8% of platinum-192 which is subject to alpha decay with a phenomenally long halflife of 10^15 years and just 0.01% of platinum-190 which is alpha active with a half life of 650 thousand million years. Altogether, 30 radioactive isotopes are known, ranging from the inverse alpha decaying platinum-168 to the beta decaying platinum-201. Claim to fame:
|
![]()