
|
76 OSMIUM Os (Greek: osme = smell)
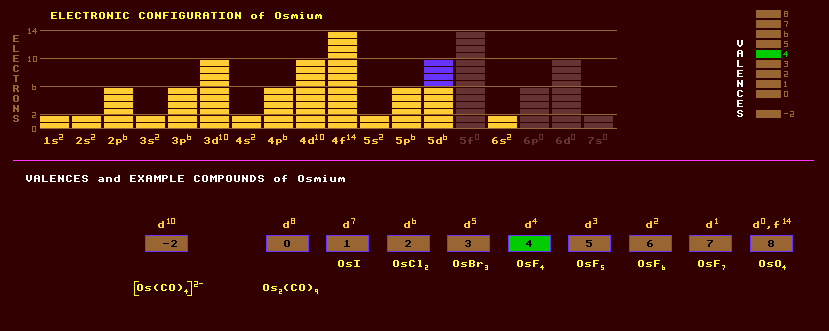
Osmium is a lustrous, bluish white, extremely hard metal of the platinum group, having the highest melting point and lowest vapour pressure of that group. It is brittle even at extended temperatures. It is extremely heavy, being the densest element, and is unaffected by air, water, and acids, but is dissolved by molten alkalis. It is named after its obnoxious smell it evolves when heated in air, due to the formation of the volatile and highly poisonous osmium tetroxide, OsO4, a powerful oxidising agent. Osmium tetroxide has been used to stain fatty tissue for electrom microscopy. The high density of osmium and iridium is due to the poor shielding of the high positive nuclear charge by the f-orbital electrons which are slowly filled in the lanthanide series. The f-orbitals have many lobes with large gaps allowing the nucleus to pull the outer electrons of the transition metal elements that follow them towards the nucleus, thus making them small, and endowing upon them high density. This also accounts for their high ionization energies (esp. mercury) and low chemical reactivity (esp. gold and platinum). Measurements suggest that osmium is slightly more dense than iridium, but calculations suggest the other way round, with iridium being 22.65 and osmium only 22.61. Interestingly, the crystal structure of osmium is hexagonal close packed whereas that of iridium is the more loosely packed face centred cubic. Osmium exhibits a very wide range of valences from -2 to 8 all inclusive, examples being OsI, OsI2, OsI3, OsF4, OsF5, OsF6, and OsF7, and forms the following oxides OsO2, OsO3 and OsO4. Many carbonyl compounds and complexes are known, including Os2(CO)9. Osmium is found naturally in both the free state in platinum bearing sands, and also alloyed with iridium in osmiridium, (Os,Ir), a very hard, white alloy also containing smaller amounts of platinum, ruthenium and rhodium. Osmiridium is used for pen nibs because it is so extremely hard. Osmium is obtained commercially as a by product of nickel refining where it occurs in trace amounts. Alloyed with other platinum group metals, osmium is used almost exclusively to make extremely hard alloys for use as gramophone needles (for 78's), instrument pivots and electrical contacts. Natural osmium is slightly radioactive and occurs as a mixture of six stable isotopes and one slightly radioactive isotope. Of the stable isotopes, the most abundant is osmium-192 at 41%, followed by osmium-190 at 26%, with lesser proportions of osmium-189, osmium-188, osmium-187, and osmium-184 at just 0.02%. The radioactive isotope osmium-186 comprises 1.6% of natural osmium and is subject to alpha decay with a phenomenally long halflife of 2 × 1015 years. Altogether, 29 radioactive isotopes are known, ranging from the inverse alpha decaying osmium-162 to the beta decaying osmium-196. Claim to fame: Iridium or osmium is the most dense element, with a density of 22.6 grams per cubic centimetre.
|
![]()