
|
75 RHENIUM Re (Latin: Rhenus = Rhine)
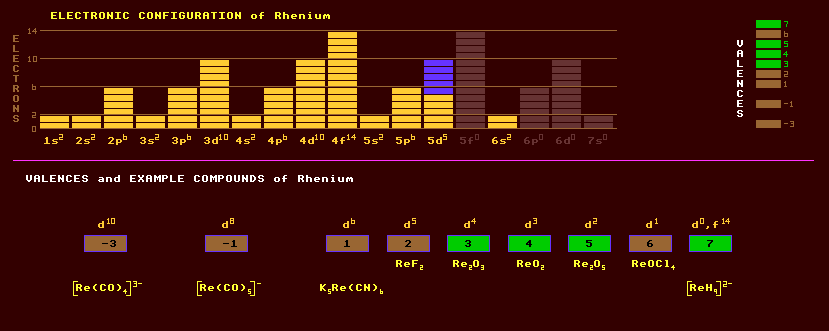
Rhenium is a very heavy, extremely hard wearing silvery metal belonging to group 7 which comprises manganese, technetium and itself. It is usually obtained as a grey powder. Rhenium resists corrosion and oxidation but slowly tarnishes in moist air. It dissolves in nitric and sulphuric acids. It has a high density exceeded only by platinum, iridium and osmium and a melting point surpassed only by carbon and tungsten. Annealed rhenium is ductile and can be bent. Rhenium is used as a catalyst, often alloyed with platinum, for the hydrogenation of chemicals and for petroleum cracking because it has exceptional resistance to poisoning by nitrogen, sulphur or phosphorus. The melting point of rhenium of 3453 Kelvin is third only to tungsten and carbon and is used in electrical filaments for mass spectrometers and ion gauges. Rhenium is used in electrical contacts because it has good wear resistance and withstands arc corrosion. Rhenium wire is used in photoflash lamps for photography. Rhenium is added to some molybdenum-based and tungsten based alloys to impart useful properties. Rhenium-molybdenum alloys are superconducting below the Curie temperature of 10 Kelvin. Rhenium is used as a thermocouple wire when paired with tungsten for measuring temperatures up to 2200 Celsius. Rhenium exhibits a large range of valences, -3, -1, 1, 2, 3, 4, 5, 6 and 7. Rhenium, like technetium, can exhibit a quadruple bond between two atoms of rhenium in the complex compound, [Re2Cl8]2-. In this instance, the quadruple bond is composed of a s-bond two pi-bonds and a d-bond. The following oxides are known: the volatile Re2O7 formed when the metal or its compounds are heated in air. This oxide dissolves in water forming perrhenic acid, from which perrhenates are formed, analogous to permanganates. The trioxide ReO3 is the anhydride of rhenic acid H2ReO4, the dioxide, ReO2, and the sesquioxide Re2O3. Many carbonyl compounds and complexes are known, including Re2(CO)10. Rhenium has been found in platinum ores and in the niobium ore, columbite, in the rare earth ore gadolinite, and in molybdenite, MoS2, at up to 0.2% concentration. It is commercially extracted from the flue dusts which result from the smelting of molybdenite (which is obtained from copper sulphide ores). Rhenium is very rare and does not occur free in nature, but is widely disseminated throughout the Earth at a concentration of 0.001 ppm. In 1992 it was found occurring as rhenium sulphide crystals, which is soft and light-grey, in the rubble of the Kudriavy volcano, which remains the only known source of this mineral, named rheniite. Moreover, this rheniite is the only known mineral of rhenium un-contaminated by other metals. Fumes of vapourous rhenium sulphide are issuing from volcanic vents and fumaroles of this volcano at concentrations many times higher than for any other volcano. Natural rhenium is slightly radioactive and occurs as a mixture of 2 isotopes, the most abundant of which is slightly radioactive, the isotope rhenium-187 which represents 63% of rhenium and is subject to beta decay with a halflife of 42 thousand million years, roughly two or three times the age of the Universe. The rest, 37%, consists of the stable isotope rhenium-185. Altogether, 31 radioactive isotopes are known, ranging from the inverse beta/alpha decaying rhenium-161 to the beta decaying rhenium-192. The great halflife (42,000 million years) of the beta decaying rhenium-187 is a result of the very small difference between the mass of rhenium-187 itself, and its daughter product, which amounts to an mass difference of only 2KeV. With such a small mass difference between parent and daughter, then the surrounding orbital electron orbits can have a profound influence on the resulting stability. Indeed, if rhenium-187 is fully ionised and without any orbiting electrons, then it is no longer subject to beta decay and is instead fully stable. This is because beta decay involves the emission of an electron, and the emission of the electron is aided by the very small repulsive force of surrounding orbital electrons. In fully ionised rhenium-187 the absence of orbital electrons is sufficient to sway the fine balance of stability in favour it being fully stable. Claim to fame:
|
![]()