
|
74 TUNGSTEN W Wolfram (Swedish: tung sten = heavy stone)
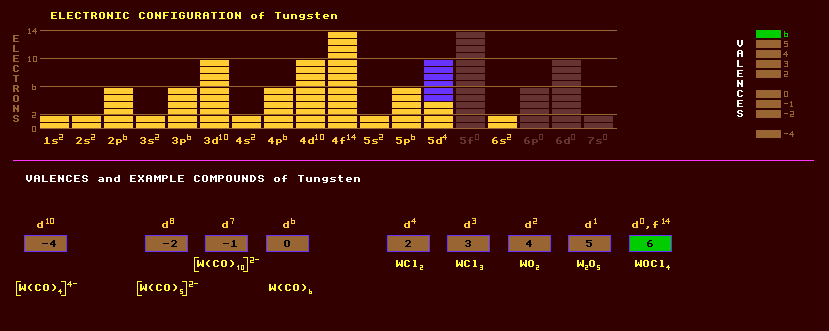
Tungsten is a hard, grey brittle group 6 transition metal element with a high strength and bulk modulus. In ultra pure form, tungsten is not brittle, but ductile. Except for carbon, tungsten has the highest melting point (3410ºC) of any element, and this made it a suitable replacement for carbon in incandescent filaments for light bulbs, halogen lamps (quartz iodide lamps), where temperatures of 2500 Celsius can be attained, taking advantage of its fairly high, for a metal, electrical resistivity. Also used as the thermionic heater in electronic vacuum tubes or valves. Tungstens' high density of 19.3 and finds use as added weight in slim darts. Tungsten has a thermal expansion coefficient close to that of borosilicate glass, and is used as glass to metal seals. Tungsten burns in oxygen at red heat to form WO3. Tungsten exists in two cubic forms, alpha and beta. Added to steels it produces high speed steels for drill bits, and Carboloy (WC + 10% Co). Other important very hard sintered alloys are Kennametal (WTiC2), Hastelloys and Stellite (Co, Cr, W). Tungsten carbide, WC, a very hard refractory material, is used to tip tungsten drill bits. Tungsten alloy is an alloy containing tungsten, copper and nickel, which is 50% denser than lead and used as protection from ionizing radiation. Tungsten exhibits a large range of valences +2 to the common +6 and has a tendency to form complex polyacids as in paratungstic acid, H10W12O41 (=12WO3+H2O), metatungstic acid (H2W4O13 (=4WO3+H2O), colloidal tungstic acid (H2WO4)7, alpha tungstic acid H2WO4,H2O, beta tungstic acid H2WO4, and complex heteropolyacids with other elements like phosphorus, boron and silicon. Calcium and magnesium tungstates are used as fluorescent phosphors in fluorescent lamps. Tungsten bronzes and tungsten blue are pigments in paints. Tungsten disulphide is used as a high temperature dry lubricant at up to 500 Celsius. Compounds of tungsten make excellent dyes and are also used to impregnate clothing to make them fireproof. The most important ore of tungsten is wolframite, an iron manganese tungstate, (Fe,Mn)WO4, which forms a continuous series, with huebnerite, manganous tungstate, MnWO4 at one extreme and ferberite, iron tungstate, FeWO4 at the other. Tungsten metal is obtained by reduction of the ore with hydrogen and charcoal. Other known minerals include scheelite, calcium tungstate, CaWO4; tungstite, tungsten sulphide, WS2; wolfram ochre or tungsten acid, WO3; and stolzite, lead tungstate, PbWO4. Tungsten exists as a mixture of five stable isotopes, the rare W-180, W-182, W-183, W-184, and W-186, the last four in very roughly equal proportions. Claim to fame: Tungsten has the highest boiling point, 5660 Celsius, the highest latent heat of vaporisation (799KJ/mol), the highest cohesive energy (859KJ/mol), and the highest latent heat of atomization (849KJ/mol) of any element.
|
![]()