
|
73 TANTALUM Ta (Greek: Tantalos, father of Niobe)
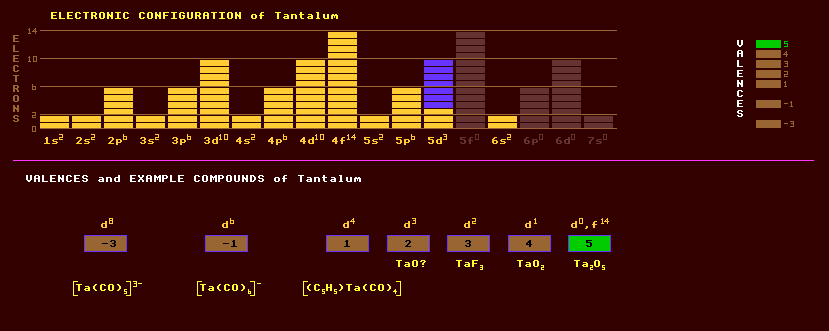
Tantalum is a shiny, very hard but soft and ductile when pure, heavy, silvery metal, belonging to group 5 comprising vanadium, niobium and itself. Tantalum is very electro-positive and would be very reactive but for the thin protective film of oxide that immediately forms on any fresh surface, conferring great corrosion resistance. The metal is attacked by hydrogen fluoride and fused alkalis. It is used as a platinum substitute in corrosion resistant laboratory apparatus, and in surgical implants because it is non-irritant and completely immune to body fluids. Alloyed with various metals it imparts high strength, high melting point and good ductility, etc, for use in chemical process equipment, nuclear reactors, aircraft and missile parts. Tantalum is used in the plates and grids and as a getter in high frequency thermionic valves. It has a melting point exceeded only by tungsten and rhenium and is used as a filament for evaporating metals such as aluminium (vacuum coating). A composite material of tantalum carbide and graphite is one of the hardest materials known and has a melting point of 3738 Celsius. Tantalum is used in dry electrolytic tantalum capacitors, which exploit the good dielectric properties of tantalum oxide. Tantalum oxide makes good rectifier diodes and is also used in making high refractive index glass for camera lenses. The oxide is very insoluble in water. Tantalum exhibits many valences: -3, -1, 1, 2, 3, 4, and 5. The following oxides are known: TaO?, TaO2, Ta2O5 and [Ta6O19]8-. The halides include TaF3, TaCl4, and TaCl5. Many carbonyl compounds and complexes are known including [Ta(CO)5]3-. Many tantalates (and niobates and titanates) crystallize in the anisotropic perovskite structure and generally exhibit a range of electro-active phenomena. Thus lithium tantalate, LiTaO3, is both piezoelectric and ferroelectric with a Curie temperature of 665 Celsius, see lithium niobate under niobium. The main ore of tantalum is tantalite, (Fe,Mn)Ta2O6, which is usually found in association with niobite, (Fe,Mn)Nb2O6, where niobium substitutes tantalum. These two minerals form a continuous range with varying proportions of niobium and tantalum called columbite, (Fe,Mn)(Nb,Ta)2O6. In some varieties manganese completely replaces replaces iron forming manganotantalite, MnTa2O6. Tantalum is obtained mostly as a byproduct of tin extraction. Other minerals include betafite, (Ca,Na,U)2(Nb,Ta)2O6(OH) and microlite, (Na,Ca)2Ta2O6(O,OH,F), stibiotantalite SbTaO4, tapiolite Fe(Ta,Nb)2O6, euxenite (Y,Er,Ce,La,U)(Nb,Ti,Ta)2(O,OH)6, and polycrase (Y,Er,Ce,La,U)(Nb,Ti,Ta)(O,OH)6, with the last two forming a continuous series. Tantalum consists almost entirely of one stable isotope, tantalum-181. Tantalum is slightly radioactive due to the presence of a little, 0.01%, tantalum-180, which has a phenomenally long halflife of over 10^13 years, much much longer than the age of the Universe. This isotope has a dual mode decay, decaying by inverse beta decay or by beta decay. Altogether, 30 radioactive isotopes of tantalum are known, ranging from the inverse beta decaying tantalum-156 to the beta decaying tantalum-186. Claim to fame:
|
![]()