
|
72 HAFNIUM Hf (Latin: Hafnia = Copenhagen)
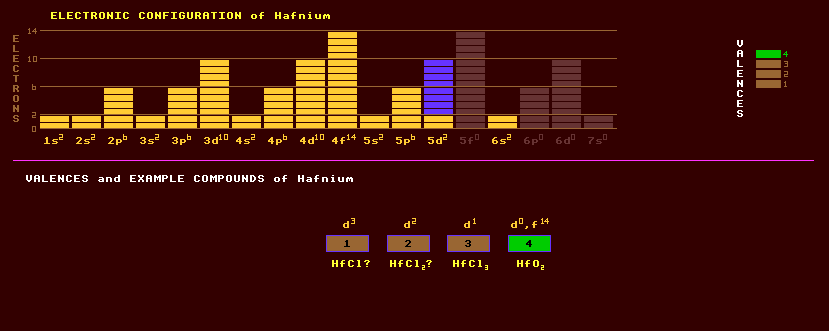
Hafnium belongs to group 4 comprising titanium, zirconium and itself. It is a very heavy, lustrous, silvery and ductile metal which resists corrosion due to the formation of a protective oxide film, but powdered hafnium will burn in air, finely divided hafnium is pyrophoric and will ignite spontaneously in air. Hafnium is considerably affected by any zirconium impurities. It is unaffected by alkalis and by acids except for hydrogen fluoride. At elevated temperatures, hafnium will react with oxygen, nitrogen, carbon, boron, sulphur, and silicon. Hafnium is used as an efficient getter for scavenging oxygen and nitrogen in thermionic valves. In sponge hafnium at 700 Celsius, each hafnium atom will absorb 1.86 times as many atoms of hydrogen. Being extremely in-soluble it's dissolved concentration in water is very low. It is alloyed with tungsten in incandescent filaments to prevent the re-crystallization of the tungsten. Hafnium has also been alloyed with iron, titanium, niobium, tantalum, and other metals. Hafnium exhibits valences from 1 to 4 inclusive. Examples include HfCl, HfCl2, HfCl3, HfO2, and HfF4. Hafnium carbide is the most refractory binary compound known. Hafnium nitride is the most refractory of all known metal nitrides, with a melting point of 3310 Celsius. Some hafnates (and many tantalates, niobates and titanates) crystallize in the anisotropic perovskite structure and generally exhibit a range of active phenomena. Thus lead hafnate, PbHfO3, is anti-ferroelectric with a Curie temperature of 215 Celsius, see lithium niobate under niobium. Hafnium, due to its chemical similarity to zirconium, occurs in association with several zirconium oxide and silicate ores. The main ore of hafnium is alvite, (Hf,Th,Zr)SiO4.xH2O, but the main source of hafnium is as a by product of zirconium refining. Zirconium and hafnium are the most difficult of all the elements to separate because of their chemical similarities, but zirconium is half the density of hafnium. Due to the relatively high neutron absorption of hafnium (600 times higher than zirconium), hafnium is a troublesome impurity of zirconium metal (chosen for its low neutron absorption) which is used to contain nuclear fuel in reactors. Because of its relatively high thermal neutron absorption, hafnium finds use as control rods in nuclear reactors. An ultra-high temperature ceramic based on hafnium diboride, HfB2, can withstand temperatures up to 2800 Celsius, its' melting point, but is extremely brittle. It may in future be used as heat shielding tiles on space vehicles for protection against re-entry heat. It can withstand much greater temperatures than those used on the space shuttle, allowing the design of more aerodynamically shaped spacecraft. Hafnium exists as a mixture of five stable isotopes, the most abundant of which is hafnium-180 at 35%, followed by hafnium-178 at 27%, hafnium-177 at 19%, hafnium-179 at 14%, and hafnium-176 at 5%. Hafnium is slightly radioactive due to the presence of a sixth and slightly radioactive isotope, hafnium-174 present at 0.2%, which is subject to alpha decay with the phenomenally long halflife of 2000 Tera years. Altogether, 26 radioactive isotopes are known, ranging from the inverse beta decaying hafnium-154 to the beta decaying hafnium-184. Claim to fame:
|
![]()