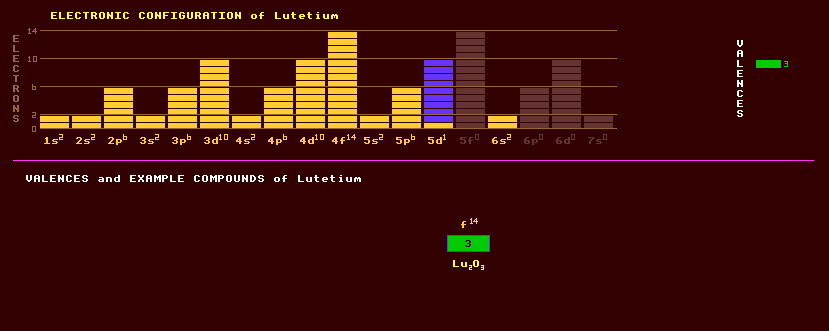

|
71 LUTETIUM Lu (Latin: Lutetia = Paris)
Lutetium is a trivalent rare earth metallic element being the hardest and densest of the lanthanide series. The spelling of this element was changed from lutecium to lutetium in 1949, but sometimes called cassiopeium by the Germans. It is one of the rarer lanthanides, slightly more abundant than thulium. The metal is relatively stable in air. Lutetium is present in very small amounts in nearly all minerals containing yttrium, and is present in monazite, a principal source, to the extent of 0.003%. Lutetium, like other rare earths, has a low toxicity, but should be handled with care. Natural lutetium is radioactive, and consists of two isotopes, 97.4% of the stable lutetium-175 and 2.6% of the unstable, beta decaying lutetium-176 which is radioactive with a half life of 30 thousand million years. Stable lutetium isotopes, which emit pure beta radiation after thermal neutron activation, can be used as catalysts in cracking, alkylation, hydrogenation and polymerization. Virtually no other commercial uses of lutetium have yet been found, as it is still one of the most costly natural elements. A total of 36 radioactive isotopes are known, ranging from the proton dripping lutetium-151 to the beta decaying lutetium-183. Claim to fame:
|
![]()