
|
70 YTTERBIUM Yb (Ytterby, a town in Sweden)
Ytterbium is a soft, malleable, quite ductile silvery white rare earth metallic element of the lanthanide series. While the element is fairly stable, it should be kept in sealed containers to protect it from air and moisture, with which it slowly reacts. Ytterbium is readily attacked and dissolved by dilute acids.
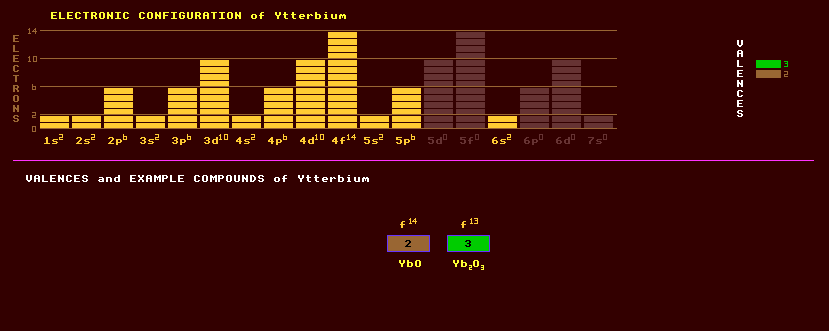
Ytterbium exists in three allotropic forms, the face centred cubic Ytterbium has possible uses in improving the grain refinement strength , and other mechanical properties of stainless steel. Ytterbium has a low toxicity. Ytterbium occurs along with other rare earths in a number of rare minerals, but is commercially recovered principally from monazite sand, (Ce,La,Nd,Th)PO4, which contains about 0.03%. Like all the lanthanide rare earths, ytterbium forms predominantly trivalent compounds, which are mostly colourless, but it also forms bivalent compounds. Natural Ytterbium is a mixture of seven stable isotopes, the most abundant of which is ytterbium-174 at 34%, followed by ytterbium-172 at 22%, ytterbium-173 at 16%, ytterbium-171 at 14%, ytterbium-176 at 13%, ytterbium-170 at 3% and just 0.1% ytterbium-168. A total of 24 radioactive isotopes are known, ranging from the inverse beta decaying ytterbium-152 to the beta decaying ytterbium-180. Claim to fame:
|
![]()