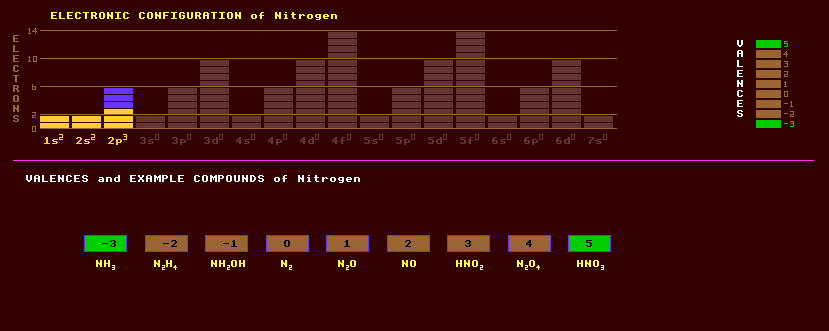

|
7 NITROGEN N (Greek: nitron genes = nitre forming)
Nitrogen is a colourless odourless diatomic gas which makes up 78.1% of the Earths atmosphere. It is quite unreactive at normal temperatures. The residence time for atmospheric nitrogen is 20 million years. Plants are highly dependant upon an easily available source of nitrogen, but cannot usually obtain it directly from the atmosphere, only certain leguminous plants can fix nitrogen directly from the air with the help of certain bacteria attached to their roots. Several oxides exist, nitric oxide, NO, is an atmospheric pollutant formed by heating air to high temperatures, such as within the internal combustion engines of cars. Nitrogen dioxide, NO2, and its dimer, N2O4, a brown gas, can be formed by passing an electrical discharge through air, as occurs during lightning strikes, and this is the natural way that compounds of nitrogen formed on Earth. This also occurs inside combustion engines and is responsible for a lot of airborne pollution. Nitrogen dioxide forms nitric acid with water, an important compound of nitrogen. Nitrogen can form many other oxides, one, nitrous oxide, N2O, or laughing gas, colourless and odourless, was once used as an anaesthetic in surgery. Other oxides are nitrogen trioxide, NO3, a bluish gas; nitrogen pentoxide or nitric anhydride, N2O5, a white solid which forms nitric acid, HNO3, with water; and nitrous anhydride, N2O3, a reddish-brown gas which forms nitrous acid, HNO2, with water. Nitrogen liquefies at -196 Celsius and is used as a cryogenic liquid. With hydrogen, under certain conditions, it forms ammonia, NH3, an obnoxious but important gas, which dissolved in water forms ammonium hydroxide, NH4OH, used as a household bleach. Ammonium nitrate, NH4NO3, is an important fertiliser for those plants unable to fix their own supply of nitrogen, unfortunately, it is also an explosive. Nitrogen is present in many alkaloids which are manufactured in plants. Potassium nitrate KNO3, and potassium nitrite, KNO3, are used as a food preservatives, and in the case of the nitrate, as an oxidant in fireworks and gunpowder. Nitrogen has found extensive use in a great variety of high explosives, of which TNT, trinitrotoluene, CH3.C6H2.(NO3)3, is just one example. Salts of hydrazoic acid, HN3, or azides, like lead azide Pb(N3)2, form initiatory explosives. The triple bond between the nitrogen atoms holds a great deal of energy. Hydrazine, N2H4 is used as a rocket fuel in spacecraft. There is also a hydrazine azide, N2H4.HN3, and ammonium azide, NH4N3. Many compounds of nitrogen are unstable and liable to explode spontaneously, like nitrogen triiodide, NI3.NH3. Nitrogen can form extremely hard highly refractive nitrides with certain metals; for instance Boron Nitride, BN, is nearly as hard as diamond. Forms compounds with sulphur, as in the unstable explosive ring compound, tetrasulphur tetranitride, N4S4. Nitrogen in the blood of deep sea divers is responsible for the (nitrogen) bends, a debilitating condition experienced when surfacing too quickly caused by the nitrogen coming out of solution and causing bubbles to appear in the blood. Nitrogen is comprised of two stable isotopes, N-14 at 99.6% and N-15 at just 0.4%.In addition, 8 radioactive isotopes of nitrogen are known, ranging from N-12 to N-21. Claim to fame:
|
![]()