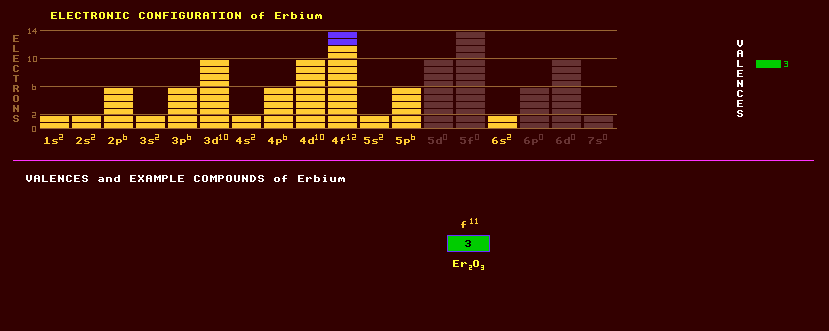

|
68 ERBIUM Er (Ytterby, a town in Sweden)
Erbium is a trivalent soft, malleable silvery grey rare earth metallic element of the lanthanide series is found in minerals mentioned under dysprosium. As with other rare earths, its properties depend to a certain extent on the impurities present. The metal is fairly stable in air and does not oxidize as rapidly as some of the other rare earths. Erbium is paramagnetic at room temperature, and becomes ferromagnetic at 41 Kelvin Erbium is finding nuclear and metallurgical uses: added to vanadium it lowers the hardness and improves workability. It is also alloyed with titanium. Below 1640 Kelvin, the crystal structure of erbium is hexagonal close packed, with two crystal axes, a and c. The opposing changes in dimensions along the a- and c-axes between 37 Kelvin and 87 Kelvin lead to an un-usual situation whereby erbium occupies almost constant volume in this range. Most of the rare earth oxides have sharp absorption bands in the visible, ultraviolet and near infrared. This property, associated with the electronic structure, gives rise to beautiful pastel colours of the rare earth salts. Erbium oxide is rose coloured and has been used as a colourant in glasses and porcelain enamel glazes, whilst its salts are a deep rose colour. Recent production techniques involving ion exchange reactions have resulted in much lower prices of the rare earths and compounds in recent years. Erbium-doped optical fibres are being used as optical fibres with in-built optical amplification throughout the length, eliminating the need for repeaters every 50 kilometres in optical communication, but, however, suffer from cumulative optical pulse distortion (whereas with repeaters, the optical pulse can be re-shaped before becoming too bad). Erbium is also used in infrared absorbing glass. Naturally occurring erbium is a mixture of six stable isotopes, the most abundant of which is erbium-166 at 34%, followed by erbium-168 at 27%, erbium-167 at 23%, erbium-170 at 15% with erbium-164 at 2% and erbium-162 at just 0.1%. A total of 21 radioactive isotopes are known, ranging from the inverse beta decaying erbium-147 to the beta decaying erbium-173. Claim to fame:
|
![]()