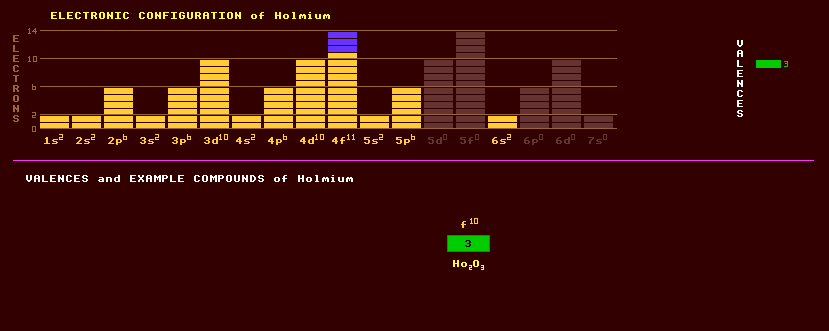

|
67 HOLMIUM Ho (Holmia = Stockholm)
A trivalent relatively soft, malleable silvery rare earth metallic element of the lanthanide series with a metallic to bright silver lustre, and stable in air at room temperature, but rapidly oxidises in moist air and at elevated temperatures. It is used as a magnetic flux concentrator for high magnetic fields. The metal, as with other rare earths, seems to have a low toxicity. Paramagnetic at room temperature, holmium becomes ferromagnetic below 85 Kelvin. Holmium occurs in gadolinite, Be2FeY2Si"O10; monazite, a lanthanide phosphate, LnPO4 from which it is commercially obtained, being present to the extent of about 0.05%; and in other rare earth minerals. It has been isolated in pure form only in recent years by ion exchange and solvent extraction techniques, and isolated by the reduction of its anhydrous chloride or fluoride with calcium metal. Holmium oxide, holmia is yellow whilst its salts are a yellow or green colour. Holmium consists of just one stable isotope, holmium-165. A total of 26 radioactive isotopes are known, ranging from the inverse beta decaying holmium-144 to the inverse beta decaying holmium-172. Claim to fame:
|
![]()