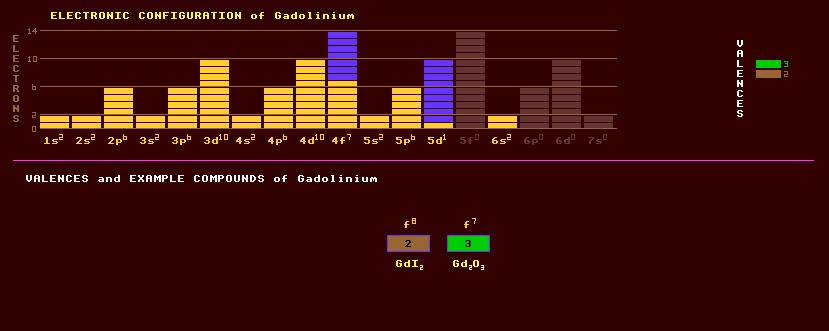

|
64 GADOLINIUM Gd (Gadolin, a Finnish chemist)
A trivalent silvery white rare earth metallic element of the lanthanide series with a metallic lustre, only known in combination, and obtained from the same sources as europium. The metal is malleable and ductile.
At room temperature, gadolinium crystallizes in the hexagonal, close-packed Gadolinium is highly paramagnetic, having the highest magnetic susceptibility, 185000, of any element. Gadolinium is unique in having a Curie temperature just above room temperature (20ºC, below which it becomes ferromagnetic) and alloyed with iron is finding use in laser-written magneto-optic re-recordable CDs and magnetic discs for computers. Man made gadolinium yttrium garnets have microwave applications. Compounds of gadolinium are used in making phosphors for colour TVs. The metal exhibits unusual superconducting properties. In metallurgy, as little as 1% gadolinium has been found to improve the workability and resistance of iron, chromium and related alloys to high temperatures and oxidation. Gadolinium ethyl sulphate has extremely low noise characteristics and may find use in maser amplifiers. The element was named from the mineral gadolinite, Be2FeY2SiO10 from which it was originally obtained. Gadolinium is found in several other minerals, including monazite, (Ce,La,Nd,Th)PO4 and bastanite which are of commercial importance. Natural gadolinium is a mixture of seven isotopes, comprising 25% gadolinium-158, 22% gadolinium-160, 21% gadolinium-156, 16% gadolinium-157, 15% gadolinium-155, 2% gadolinium-154 and just 0.2% gadolinium-152. Gadolinium has the highest thermal neutron cross sectional area of any known element of 49000 barns. The stable isotopes gadolinium-155 and gadolinium-157 present in natural gadolinium have excellent thermal neutron capture characteristics, but are present in low concentrations. As a result, natural gadolinium has a very fast burn out rate and has limited use as a nuclear control rod material. Pure gadolinium exhibits a large magneto-caloric effect, whereby a magnetic field causes the metal to become hotter. The effect is more pronounced the stronger the magnetic field. This effect can provide the basis for a solid-state refrigerator, where the metal is subjected to a repeating cycle: magnetic field present, metal heats up and then that heat is removed by water cooling so that it is at the temperature it started at. When the magnetic field is then removed the metal becomes colder that when it started. By repeating this process, the gadolinium will get colder and colder, the basis of refrigerators. But the alloy Gd5(Si2Ge2) exhibits a giant magnetocarloric effect, and is even more effective for refrigeration, and does not require expensive high-purity gadolinium.
Claim to fame: Gadolinium has the highest paramagnetic susceptibility (185,000
|
![]()