
|
63 EUROPIUM Eu (Europe)
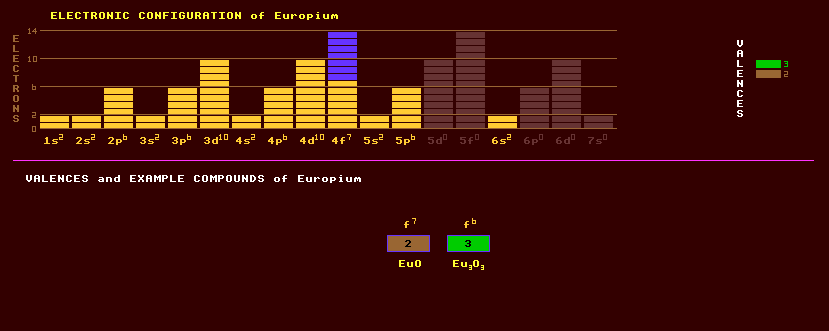
Europium is a soft, silvery metal, a rare and most reactive member of the lanthanide series of the rare earths. Europium reacts quickly with oxygen and with water. Europium is little used. Some of the metal is used in thin-film superconducting alloys. Europium, like all the lanthanide rare earths, is predominantly trivalent, but also forms divalent compounds. Examples of compounds being EuO, EuS, EuF2, EuCl2, Eu2O3, Eu(OH)3, EuF3, etc. Both europium oxide and europium salts are a pale rose colour. When europium exhibits the +2 valency, its ionic radius is anomalously large compared to other lanthanides, only slightly larger than the radius of calcium, and thus it can substitute for calcium in rocks, causing the 'europium anomaly'. A plot of the rare earth abundances in highland moon rocks shows that, of the rare earths europium is thirty times more abundant in highland moon rocks (which are predominantly CaAl2Si2O8) in relation to any other lanthanide. Under strongly reducing conditions, when most other lanthanides are confined to using the +3 valence state, europium uses the +2 state, which is similar in size and has the same +2 valency as strontium, for which it can also substitute. Thus it is occasionally also found in association with strontium. Trivalent europium and other trivalent lanthanides (holmium, gadolinium, thulium, erbium, and praseodymium) are used to dope a variety of hosts such as glass, YAG, calcium tungstate, yttrium oxide, strontium molybdate or lanthanum trifluoride in crystal form to produce solid state lasers emitting various wavelengths. Europium is contained in the ores black monazite (Ce,La,etc)PO4, bastnaesite (Ce,La,etc)(CO3)F, gadolinite Be2FeY2Si2O10, samarskite (Y,Ce,U,Ca,Pb)(Nb,Ta,Ti,Sn)2O6, and xenotime YPO4. Europium exists as just two stable isotopes, 52% of europium-153 and 48% europium-151. Twenty five radioactive isotopes are known, ranging from the inverse beta decaying europium-134 to the beta decaying europium-160. Claim to fame:
|
![]()