
|
62 SAMARIUM Sm (Samarskite, a mineral)
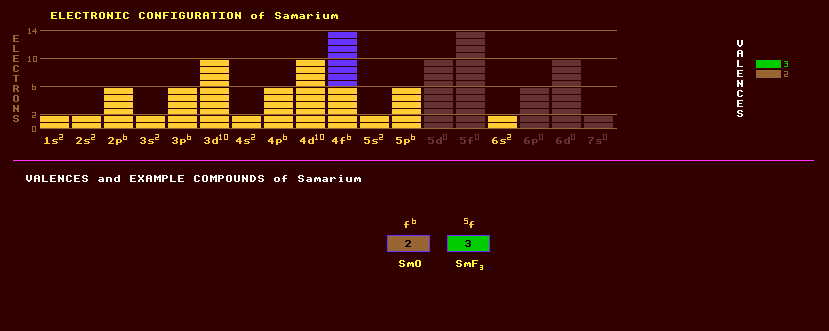
Samarium is a hard and brittle rare earth metallic element of the lanthanide series with a bright silver lustre which is reasonably stable in air. Two crystal structures of the metal exist, with a phase transition temperature of 917ºC. Samarium ignites in air at 150ºC. Whilst misch metal, an alloy of cerium, lanthanum, neodymium and praseodymium which also contains about 1% samarium, which has long been used as a pyrophoric alloy for lighter flints, samarium has only recently been isolated in relatively pure form. Finds use constituent of very strong samarium cobalt, SmCo5, magnets, which have a very high magnetic energy density and the highest coercive force, 28000 oersteds, of any known magnet. Found along with other members of the rare earths in many minerals, including monazite, (Ce,La,Nd,Th)PO4 (where it occurs to the extent of 2.8%) and bastanite, which are commercial sources, and is also found in allanite, (Ce,Ca,Y)2(Al,Fe)2(SiO4)3(OH); cerite, gadolinite, and samarskite, (Y,Ce,U,Ca,Pb)(Nb,Ta,Ti,Sn)2O8 in which it was first discovered. Samarium, like all lanthanides, is predominantly trivalent, but also forms divalent compounds. The oxide is a pale yellow colour, and the salts are topaz yellow. Samarium oxide, along with other rare earths, is a highly refractory substance and is used as a core material for carbon-arcs in the cinema industry. It is also used to dope calcium fluoride crystals for use in lasers. Compounds of the metal act as sensitisers for phosphors used in colour TVs. The sulphide has excellent temperature stability and good thermoelectric efficiencies up to 1100ºC. Samarium oxide has been used in optical glass to absorb infrared, and exhibits catalytic properties in the dehydration and dehydrogenation of ethyl alcohol. Natural samarium is a mixture of seven isotopes, three of which are slightly radioactive with long half lives, thus it is feebly radioactive. The four stable isotopes comprise 27% samarium-152, 23% samarium-154, 7% samarium-150 and 3% samarium-144. The rest comprises of three alpha decaying radioactive isotopes: 15% samarium-147 with the extremely long halflife of 108 thousand million years, 14% samarium-149 with the phenomenally long half life of 10 x 10^15 years, and 11% samarium-148 with halflife 7 x 1015 years. Samarium can be produced by decay of fission fragments within a nuclear reactor and is a nuclear reactor poison because it absorbs the neutrons that sustain the reaction. Samarium is used as a neutron absorber in nuclear reactors. Claim to fame:
|
![]()