
|
61 PROMETHIUM Pm (Greek person: Prometheus)
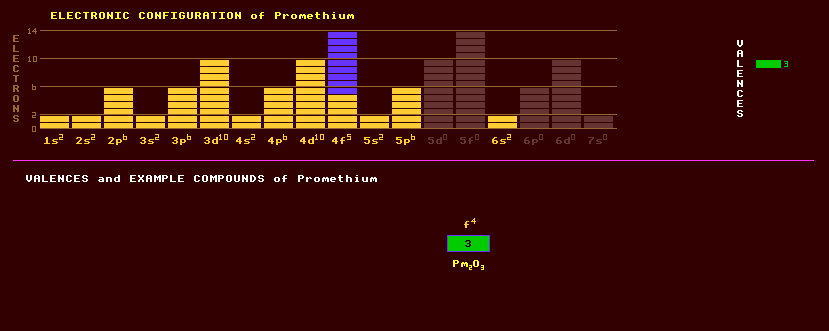
A trivalent radioactive rare earth metallic element of the lanthanide series, with no stable isotopes. Promethium, atomic number 61, is just the second element for which no stable isotopes exist, the first being technetium (atomic number 43); all other elements up to atomic number 83 (bismuth) have at least one stable isotope. In 1941 neodymium was irradiated with neutrons and deuterons, and praseodymium with alpha particles producing several new radioactive species, probably some promethium, but confirmation was lacking because of the difficulty in isolating different rare earth elements at the time. Promethium was first chemically identified in 1945 by ion-exchange chromatography upon material produced by the fission of uranium. Searches for the element on earth have proved fruitless, and it now appears that promethium is completely missing from the earth's crust. Promethium has, however, been identified in the spectrum of light from the star HR465 in Andromeda. This element must be being formed near the surface of the star, for no known isotope of promethium has a half-life greater than 17.7 years. Promethium is collected by ion exchange methods from the processing wastes of nuclear reactor fuel. Little is yet known about the properties of metallic promethium. Two or more allotropic forms are thought to exist. Promethium exhibits just one valency, +3. The following compounds have been prepared: promethium sesquioxide, Pm2O3, a hydroxide, Pm(OH)3, a trifluoride, PmF3, and the complex [Pm(H2O)x]3- which exists only in aqueous solution. More than thirty promethium compounds have been prepared, most are coloured. Promethium salts luminesce a pale blue or green due to radioactivity. Promethium is on of many fission products produced by fission reactions within nuclear reactors and occurs in the fallout from nuclear explosions. Promethium-147, with a half-life of 2.5 years, is the most generally useful. Promethium-145 is the longest lived, and has a specific activity of 940 Curie per gram. It is a soft beta emitter, and although no gamma rays are emitted, X-radiation can be produced when beta particles impinge on elements with a high atomic number, and great care must be taken in handling it. Promethium salts luminesce in the dark with a pale blue or greenish glow, due to their high radioactivity. Promethium-145 is used as a beta source for thickness gauges and for luminescent signs where the beta rays strike a phosphor producing light, that light can also generate electricity in a photocell giving a nuclear battery a useful life of 5 years. Promethium could be used as a portable X-ray unit, and as a heat source in thermopiles to provide auxiliary electrical power for space probes and satellites. Thirty isotopes of promethium are known, all radioactive, from Pm-128, which decays by inverse beta decay with a halflife of 2.2 seconds to Pm-158, a beta emitter with a halflife of 5 seconds. The longest lived isotope of promethium is promethium-145 with a halflife of 17.7 years decaying by alpha decay into stable praseodymium-141 or by inverse beta decay into stable neodymium-145. Claim to fame: Promethium (At No 61) seems to be the only element below the atomic number of 95 that is completely missing from the Earths crust.
|
![]()