
|
60 NEODYMIUM Nd (Greek: neos didymos = new twin)
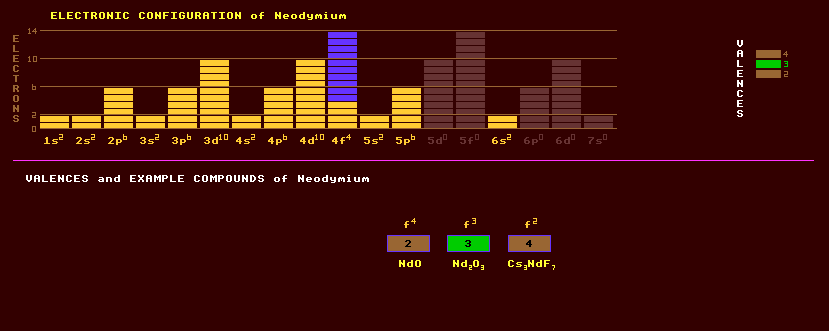
A trivalent silvery white rare earth metallic element of the lanthanide series originally found in cerite, as the rose coloured oxide. Neodymium is one of the more reactive rare earth metals and quickly tarnishes in air, forming an oxide layer which spalls off exposing new metal to air. The metal should therefore be kept under light mineral oil or otherwise sealed off. Neodymium exists in two allotropic forms, with a transformation from a double hexagonal to a body-centred cubic structure at 860ºC. While neodymium is present to the extent of 18% in misch metal (an alloy of cerium , lanthanum, praseodymium and neodymium), long known and used as a pyrophoric alloy for lighter flints, the element was not isolated in relatively pure form until 1925. It has found recent use in very powerful magnets when alloyed with other metals like iron. It is present in the minerals monazite, (Ce,La,Nd,Th)PO4 and bastanite, which are principal sources of the rare earth metals. It is also obtained from bastnaesite, (Ce,La,etc)(CO3)F. It is also found in cerite and orthite (allanite), (Ca,Ce,Y,La,Th)2(Al,Fe)3Si3O12(OH). Neodymium oxide, is light blue, whereas the salts are red-violet. Neodymium exhibits three valences, 2, 3 and 4. Two oxides are known, NdO and Nd2O3. Other compounds include Cs3NdF7, NdCl3 and NdI2. By itself, neodymium colours glass delicate shades ranging from pure violet through wine-red and warm grey. Light transmitted through such glass shows unusually sharp absorption bands, and is useful in astronomical work to produce sharp bands by which other spectral lines may be calibrated. Glass containing neodymium, called neodymium glass, is used as a lasing material in place of ruby to produce coherent light. Neodymium salts are used as a colourant for enamels and ceramics. Didymium, once thought to be a new element but now known to be a mixture of neodymium and praseodymium, is used for colouring glass to make welders goggles. Neodymium is used to dope glass and yttrium aluminium garnet (YAG crystals) to make very powerful infrared lasers. The most powerful neodymium glass laser in the World generates 100 TeraWatt pulses of green or blue laser light lasting just 1 nanosecond to induce fusion in a tiny target of deuterium and tritium. The green and blue wavelengths are obtained by passing the infrared beam through an optically non-linear filter such as KDP or potassium dihydrogen phosphate which generates the second and third harmonics of the infrared beam, which lie in the green and blue parts of the spectrum. Neodymium has a low to moderate toxicity, and, as with other rare earths, should be handled with care. Natural neodymium is slightly radioactive being a mixture of six stable isotopes and a high proportion (24%) of a slightly radioactive beta decaying slightly isotope, neodymium-144 which has the phenomenally long halflife of 2.1 x 10^15 years. The stable isotopes comprise 27% neodymium-142, 17% neodymium-146, 12% neodymium-143, 8% neodymium-145 ,6% each of neodymium-148 and neodymium-150. Altogether a total of 25 radioactive isotopes are known, ranging from the inverse beta decaying neodymium-127 to the beta decaying neodymium-156. Claim to fame:
|
![]()