
|
58 CERIUM Ce (Ceres - an asteroid)
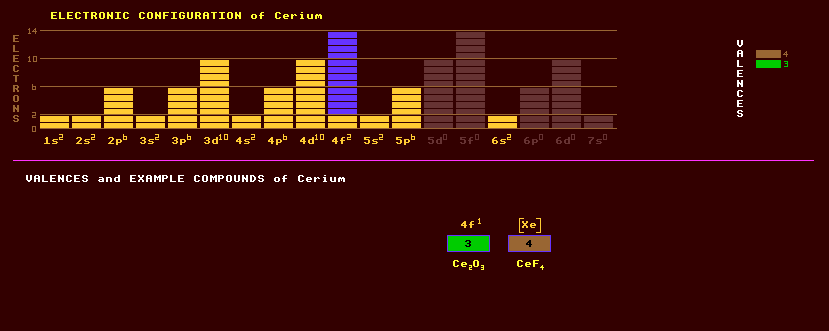
An iron-grey malleable lustrous lanthanide series rare earth metallic element which oxidizes very readily at room temperature, especially in moist air. Except for europium, cerium is the most reactive of the rare earth metals, it slowly decomposes in cold water, and rapidly in hot water. It is readily attacked by alkalis and dilute acids. The pure metal is likely to ignite if scratched with a knife. Pure cerium is not radioactive, but commercial grade may contain traces of thorium, which is radioactive. Cerium, with other rare earths, is used in cinema carbon-arc lighting and as an important catalyst in petroleum refining. Cerium is especially interesting because of its variable electronic structure. The energy of the inner 4f electron is nearly the same as that of the outer or valence electrons; only small amounts of energy are required to change the relative occupancy of these levels. This gives rise to dual valency states. A volume change of about 10% occurs when cerium is subjected to high pressures or low temperatures. The valency changes from 3 to 4 when it is cooled or compressed.
The low temperature behaviour of cerium is complex. Four allotropic modifications are thought to exist: cerium at STP is known as Along with lanthanum, praseodymium and neodymium, cerium is a constituent of the alloy misch metal, used as a pyrophoric metal in cigarette lighter flints. Like all lanthanide rare earths, cerium is predominantly trivalent, but also forms tetravalent compounds. The ceric salts are orange red or yellowish, the cerous salts are usually white, as is the oxide. The oxide, ceria, is the luminous constituent of incandescent gas mantles, and is used on the walls of 'self-cleaning' ovens as a hydrocarbon catalyst. Ceric sulphate finds extensive use as a volumetric oxidizing agent in qualitative analysis. Cerium compounds are used in the manufacture of glass, both as a component and as a decolourant. The oxide is used to polish glass instead of rouge because it is faster. Cerium fluoride is used as one of the anti-reflective coatings on multi-coated camera lenses etc. Heavy fermion compounds are compounds that contain such rare earth elements as Cerium or Ytterbium, or actinide elements such as Uranium. Examples are CeRu2Si2, CeCu6, YbCuAl and URu2Si2, UPt3. The f-electrons are nearly localised in atomic-like configurations. The motion of these itinerant electrons is highly correlated due to the strong Coulomb interaction between electrons in the same ionic shell. This results in large effective quasi-particle masses which can be up to 100 times greater than for normal rare earth and actinide compounds. At low temperatures, some of the materials are magnetically ordered, others strongly paramagnetic, and some display superconductivity at high pressures (URu2Si2, UPt3). Cerium is the most abundant of the rare earth metals, and is found in a number of minerals including allanite (orthite), cerite, samarskite, monazite and bastanite, the latter two are the most important sources of cerium. Cerium exists as four stable isotopes, 88% cerium-140, 11% cerium-142, and about 0.2% each of cerium-136 and cerium-138. A further 26 radioactive isotopes are known, ranging from the inverse beta decaying cerium-123 to beta decaying cerium-152. Claim to fame: Cerium has the lowest temperature coefficient of resistance of any metallic element (870ppm/ºC).
|
![]()