
|
57 LANTHANUM La (Greek: Lanthanein = to lie hidden)
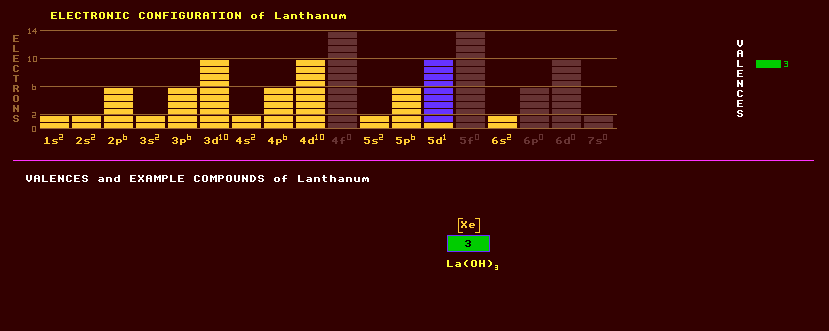
A trivalent soft malleable, ductile silvery white rare earth metallic element of the lanthanide series (obviously). It is soft enough to be cut with a knife. Lanthanum is one of the most reactive of the rare earth metals, and oxidises rapidly when exposed to air. Cold water attacks lanthanum slowly, but hot water attacks it much more rapidly, producing hydrogen gas. The metal reacts directly with elemental carbon, nitrogen, boron, selenium, silicon, phosphorus, sulphur, and with the halogens. At 310 Celsius lanthanum changes from a hexagonal form to a face centred cubic structure, and at 865 Celsius it again transforms into a body centred cubic structure. Misch metal, an alloy of cerium, lanthanum, neodymium and praseodymium, is used in lighter flints, in which it occurs at 25%. Hydrogen sponge alloys containing lanthanum can absorb up to 400 times their own volume of hydrogen gas; the process is reversible and liberates heat in the process. Possible applications include the relatively safe storage of hydrogen fuel for vehicles. Lanthanum exhibits just one valency, that of 3. The known compounds include La2O3, LaF3, LaOCl, [La(NCS)6]3- and complexes. A lanthanum hydride is known. Lanthanum is found in rare earth minerals such as cerite, allanite, (Ce,Ca,Y)2(Al,Fe,)3(SiO4)3(OH); monazite, (Ce,La,Nd,Th)PO4; and bastanite, the last two of which are principal ores in which lanthanum occurs in percentages up to 25% and 38%, respectively. Rare earth compounds containing lanthanum are extensively used in carbon arc lighting applications, especially by the cinema industry. Lanthanum oxide, La2O3, improves the resistance to alkali attack of glass, and is used in making special high refractive index low dispersion photographic lenses. Small amounts of lanthanum, as an additive, can be used to produce nodular cast iron. Lanthanum Manganate, LaMnO3, is an antiferromagnetic insulator which exhibits the 'collosal magnetoresistance' effect', where the electrical resistance increases suddenly as the temperature is increased through its magnetic Curie temperature. This 'collosal magnetoresistance' effect is even more pronounced than the same kind of effect in so-called 'giant magnetoresistive' compounds, which are used as magnetic read heads in computer storage systems. Natural lanthanum is slightly radioactive containing a mixture of two isotopes, the vast majority being the stable lanthanum-139 at 99.9% with just 0.09% of the dual mode beta/inverse beta decaying lanthanum-138 which has a halflife of 106 thousand million years, much longer than the age of the Universe. Altogether, 28 radioactive isotopes are known, ranging from the inverse beta decaying lanthanum-121 to the beta decaying lanthanum-149. Claim to fame:
|
![]()