
|
56 BARIUM Ba (Greek: barys=heavy)
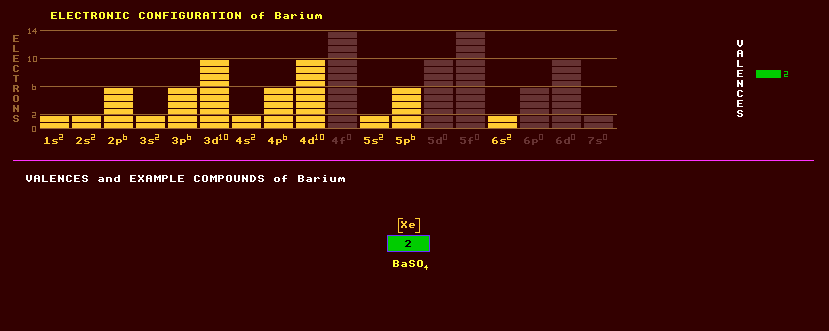
Barium is a heavy, divalent, relatively soft, silvery-white metal belonging to the alkaline earth group 2 and chemically resembles calcium. The metal readily oxidises in air and is used as a getter in thermionic valves where it coats the inside of the glass a silvery colour. If this colour changes to white, all the getter has been used up and oxidised.. Barium exhibits just a single valency, that of 2. The known compounds include a hydride BaH2, oxide BaO, peroxide BaO2, carbonate BaCO3 and sulphate BaSO4. Barium is a strong lithophile and is a widespread, but minor constituent of many silicate and other oxide minerals. The white coloured barytes, or barite, or heavy spar, barium sulphate, BaSO4, is commonly associated with lead ores. It was once thought to be useless, so was discarded when mining lead, but now finds use as drilling fluid in oil exploration, because it is so heavy. It is also used in paint and lake pigments, as filling for paper, in the manufacture of glazes and enamels, and in the making of barite cement and heavy concrete for protection against radioactivity, particularly in the construction of nuclear power stations. Because barium absorbs X-rays, a barium meal, consisting of barium sulphate, is swallowed in radiometry to outline the alimentary tract. Barytes is thermoluminescent; when heated it glows in the dark and is one of the most water-insoluble substances known. Barium is not essential to most life forms, but is present in some sea organisms for use either as ballast or as part of a gravity sensor. Baritocalcite, BaCO3.CaCO3, a double carbonate of calcium and barium, also occurs with lead ores. Barium titanate is a crystalline ceramic with exceptionally high dielectric constant which is used as the insulator in Hi-K ceramic capacitors. It has a high piezoelectric constant making it useful in ultrasonic transducers and ceramic pickup cartridges, and a high ferroelectric constant useful in pyrometers and infra-red sensing burglar alarms. It replaces Rochelle salt in gramophone pickups, having a substantially higher Curie point. Barium oxide, BaO, is more reactive with water than calcium oxide, forming a white alkali, barium hydroxide, Ba(OH)2, or baryta. Barium salts colour a flame green; barium nitrate, Ba(NO3)2, and barium chlorate, Ba(ClO3)2, are used in fireworks as both oxidant and colourant. Barium carbonate is used as a rat poison. Barium peroxide, BaO2, is an oxidant. Han purple, created by the Chinese in the Han dynasty, is a purple pigment of barium copper silicate, BaCuSi2O6, when cooled down to near absolute zero goes into an un-usual Bose Einstein condensate, where only the spins condense not the atoms or molecules themselves. Such spin condensates could be used in spintronic devices. Witherite, barium carbonate, BaCO3, which is fluorescent under ultraviolet light, is an important source of barium occurring with galena, and occurs in the lead mines of Alston Moor near Hexham. Barium titanium silicate, BaTi(SiO3)3, is strongly pleochroic, appearing sapphire blue from certain crystal directions and clear from others, and is fluorescent under UV light and used as a gemstone. A single atom of barium, called 'Astrid', has been held stationary in a three laser beam trap for the purpose of studying the properties of the element unaffected by neighbouring atoms. Despite being far beyond the resolution of the eyes, it can nevertheless be seen with the naked eye as a bright speck. Natural barium is a mixture of seven stable isotopes, the most abundant being Ba-138 present at 71%, followed by Ba-137 at 11%. The rest are Ba-136, Ba-135, and the least abundant Ba-132 and Ba-130 present at only 0.1%. A further 25 radioactive isotopes are known ranging from the inverse beta decaying Ba-117 to the beat decaying Ba-149. Claim to fame:
|
![]()