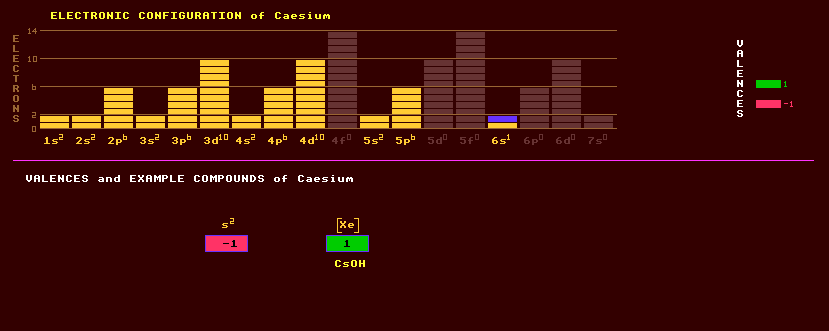

|
55 CAESIUM Cs Caesius:Latin for sky blue.
Caesium is a soft, shiny, ductile gold-coloured and very rare alkali metal, rarer than rubidium. Caesium has two characteristic bright blue lines in its spectrum. Caesium is liquid at room temperature, with a melting point of only 28.4 Celsius. A thin film of caesium coated onto a thin film of caesium oxide supported on silver is used as a photoelectric cell and as a photocathode in photomultiplier tubes (Photoconductor compound S20 is NaKCsSb). Caesium is used as a catalyst in the hydrogenation of certain organic compounds and also in atomic clocks which are accurate to within 1 second in a million years. Because it is easily ionized and has a large atomic mass, caesium can be used in ion propulsion systems in outer space, where it is 140 times more efficient than the best known fuel.
Caesium reacts rapidly with oxygen forming caesium monoxide, Cs2O, which is red, and is used as a 'getter' in electronic valves. Caesium reacts explosively with water to form the strongest alkaline known, caesium hydroxide, CsOH, which attacks glass. With caesium being the most electropositive of elements and fluorine the most electronegative, the salt caesium fluoride is the most ionic substance known. Caesium compounds are toxic to plants and animals. Caesium occurs in pollux or pollucite, a hydrated caesium alumino-silicate, (Cs,Na)4Al4(SiO3)9.H2O, found on the island of Elba at a concentration of 20%. Traces of caesium chloride, CsCl2, are found in the mine waters of Wheal Clifford mine, Cornwall Caesium exists as only one stable isotope, Cs-133. Altogether, 39 radioactive isotopes are known, ranging from the proton emitting Cs-113 to the neutron and electron emitting Cs-148. The volatile radionuclide, caesium-137, is a notorious fission product occurring as fallout as a result of atmospheric nuclear tests. It is a beta emitter with a halflife of 30 years. The flora and fauna of those acidic upland areas of Britain where it was raining at the time are still badly contaminated with caesium-137 resulting from the explosion and subsequent fire at the Chernoble nuclear power station in Kiev, Russia in 1986. The caesium-137 contamination of the grassland in acidic soils is constantly recycled by grazing sheep preventing their sale for human meat. Because of caesiums low charge and large diameter, compounds such as caesium iodide, CsI, are more volatile than most other fission products and so are vaporised first. Iodine-131 is also a serious radioactive contaminant from nuclear mishaps, which occur all too frequently. Claim to fame: Caesium has the lowest atomic concentration of just 1022 atoms/cc, the largest atomic radius (265pm), the highest polarizability (59.6 m3??), the lowest Debye temperature (38 Kelvin), the lowest first ionization energy (3.9eV), is the softest on Mohs scale of hardness (0.2), and is the most electropositive and most alkaline of the elements. Caesium also has a greatest number of radioactive isotopes, 39 in number.
|
![]()