
|
54 XENON Xe (Greek: xenos = stranger)
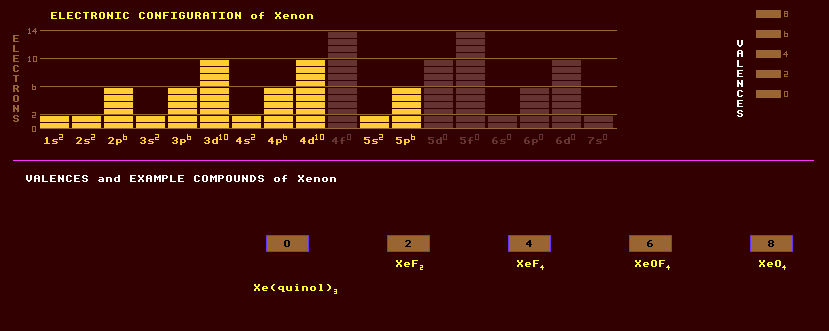
A colourless, odourless noble monatomic gaseous element once thought to be completely inert due to its completed-octet electron shell, but now known to form certain compounds with mostly fluorine or oxygen in a proliferation of even numbered oxidation states from zero to eight. It is present in certain mineral water springs, and in air at an abundance of 0.08 parts per million, from which it is obtained commercially by liquefaction followed by fractional distillation to separate it from other gases. Like hydrogen, xenon becomes metallic in character under extreme pressure. The compounds of Xenon include XeF2, XeF4, XeF6, XeOF4, XeOF4, the highly explosive colourless solid XeO3, the colourless gas XeO4, (XeF7)-, (XeF8)2-, [XeF5]+[AsF6]-, XePtF6, XeRhF6, Na4XeO6·8H2O, and more recently FXeN(SO2F)2 with a xenon-nitrogen bond. Clathrate compounds, where the xenon is mechanically trapped within a cage, such as Xe8(H2O)46 have also been made. The perxenates are used in analytical chemistry as oxidizing agents. Silent electric discharge lamps filled with xenon gas produce a beautiful blue glow, used in blue 'neon' signs. With a higher potential, xenon produces a green light. Xenon gas is used in photographic flashgun electric discharge tubes, where it produces a brilliant white, wide spectrum short duration flash. These tubes are also used to optically pump ruby lasers. Xenon is used as the gas in bubble chambers to detect sub-atomic particles, and other applications where its high molecular weight is of value. Being a heavy atom, xenon is also potentially useful as a gas for ion deep-space propulsion engines. Natural xenon consists of a mixture of 9 stable isotopes. Xenon has the highest number of known isotopes, numbering forty now. The unstable isotope, Xe-135, has the highest known capture cross section for thermal neutrons of 2,700,000 barns; formed as a result of radioactive decay of uranium fission fragments in a nuclear reactor, where it presents a problem being the most serious reactor poison, and may delay the restart of a reactor after a period of shutdown. Xe-133 and Xe-135 are produced by neutron irradiation in air-cooled nuclear reactors. Xe-133 has useful applications as a radioisotope. Based on the abundance of other inert gases in the atmosphere, there is 20 times less xenon gas in the Earths atmosphere than expected. The heavy inert gases, neon, argon, krypton and xenon have been trapped within the earth when the planet was first formed and have been leaking out into the atmosphere ever since. But the atmospheric abundance of xenon is 20 times below what it should be. I has now been discovered that, unlike the other inert gases, a xenon atom can replace a silicon atom in quartz when the two are together at pressures 100,000 times greater than atmospheric pressure and at temperatures of 2200 Celsius. Under such conditions, which can occur deep within the Earths crust and mantle the quartz soaks up all the free xenon atoms, trapping them within the 'quartz' lattice. Xenon is not toxic, but its compounds are highly toxic due to their strong oxidizing characteristics. Claim to fame: xenon has the greatest number of isotopes, 40 known, and also the lowest velocity of sound (170m/s) of any element.
|
![]()