
|
53 IODINE I (Greek: iodes = violet)
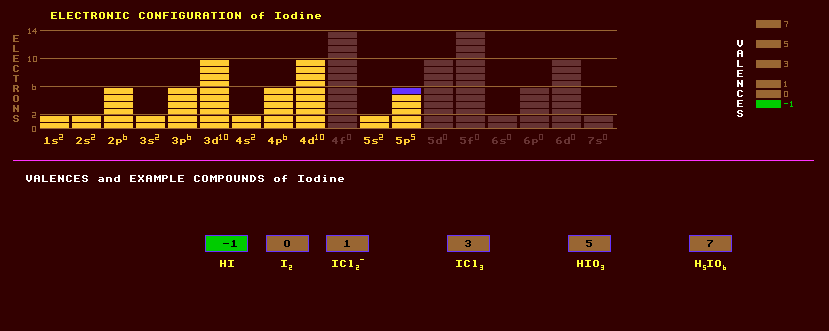
Iodine is a volatile, shiny black non-metallic solid with a violet lustre and is a member of the halogen group, group 17, after fluorine, chlorine, and bromine. It sublimes easily into a blue-violet gas with a characteristic smell. Skin contact with iodine can cause blisters. Iodine is used as a powerful disinfectant, and as a test for starch, which it stains deep purple. Iodine will dissolve in chloroform, carbon tetrachloride, carbon disulphide or forming purple solutions. Ultrapure iodine can be obtained by reacting potassium iodide, KI, with copper sulphate, CuSO4. Potassium iodide is used in photography. Iodine forms three acids: hydriodic, HI, forming iodides, and iodic acid, HIO3, forming iodates. Iodine, being a larger atom than bromine, can accommodate more oxygen atoms around it, and a periodic acid, H5IO6, is known which forms periodates. A HIO4 is also known. Iodine is less reactive than other halogens, which will displace it from iodides. Silver iodide, AgI, is used in photographic prints as the light sensitive component. Most metallic iodides liberate iodine vapour when heated. Iodine has four oxides, I2O4, I4O9, iodic oxide I2O5 which yields iodic acid in water, and IO4, all differing in marked degree to those of other halides. Unlike all other halides, iodine is oxidised by atmospheric oxygen to the iodate ion, IO3-, and small quantities are found in sodium nitrate, NaNO3. Iodine forms many interhalides: iodine heptafluoride, IF7; iodine burns in fluorine gas to form the liquid iodine pentafluoride, IF5; iodine trichloride, ICl3 which forms brown rhombic tablets and is used as a disinfectant; and iodine monochloride, ICl, which has two allotropic forms, a red acicular form and a brown-red crystalline form. Nitrogen triiodide, NI3.NH3, is a touch sensitive initiatory explosive. The biological methylation of iodine in sea water leads to a significant (2 parts in 1012) amount of methyl iodide, CH3I, in the atmosphere. Iodine is obtained from Chili saltpetre (caliche) NaNO3, and also obtained from brines, sea water and certain seaweeds. Iodine crystallizes in the orthorhombic system with three orthogonal axes, a-, b-, and c-axes. The thermal expansion of iodine is remarkable: the expansion coefficient in the c-axis is like that of metals (about 7ppm/ºC) whereas it is more than ten times that in the others (a-axis 97ppm/ºC, b-axis 113ppm/ºC) [average over -163ºC to 25ºC]. Iodine has just one stable isotope, iodine-127. Iodine-129, an electron emitter produced in trace amounts in the atmosphere by cosmic ray bombardment, has a long halflife of 17 Million years. It is also found in tellurium ores, where it is produced by cosmic ray muon bombardment of tellurium-130. Iodine-131 is a beta decaying isotope with a halflife of 8.04 days that is generated in nuclear reactors as one of the many products of uranium fission. Atmospheric tests of nuclear weapons and accidental fires in nuclear power stations release the volatile iodine where it gets into the food chain, and is strongly absorbed by the body. Iodine is an essential mineral needed by the thyroid gland which manufactures the hormone thyroxin, and the radioactive iodine can be sequestered by the thyroid if the thyroid is short of iodine. Iodine deficiency leads to goitre, or Derbyshire neck, an enlargement of the thyroid gland. Iodine tablets distributed after nuclear accidents prevent the thyroid from absorbing the radioactive variety, which can lead to cancer. Iodized salt is a recommended to ordinary salt. After two months, the contamination will have decayed to negligible proportions. The radioactive isotopes, I-131, I-132 and I-125 are used as medical tracers. Claim to fame: Iodine has the highest gaseous entropy (261 J-1 K mol-1) and the highest solid entropy (116 J K-1 mol-1) of any element.
|
![]()