
|
52 TELLURIUM Te (Latin:tellus - Earth)
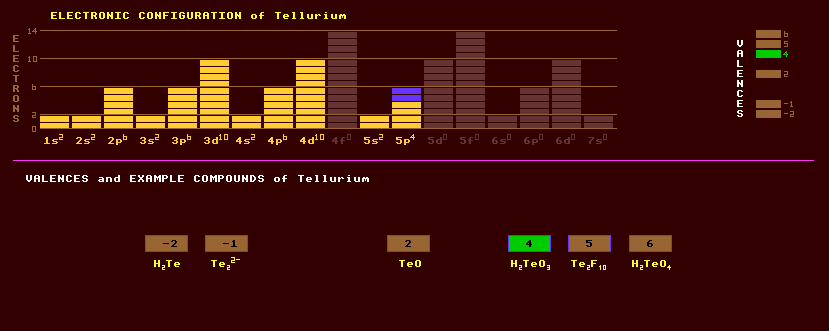
Tellurium is a semi-metal in group 16 along with oxygen, sulphur, selenium (with which it more closely resembles chemically), itself, and polonium. Tellurium is silvery white, brittle and metallic looking in bulk, but is usually obtained as an amorphous dark grey powder. Tellurium burns in air with a greenish-blue flame to become the dioxide, TeO2. Tellurium is unaffected by hydrochloric acid, but dissolves in nitric acid. Tellurium is used in the electro-refining of zinc to eliminate contamination with cobalt. It is also alloyed with lead to increase the strength of pipes and cable sheaths. Molten tellurium corrodes iron, copper and stainless steel. Tellurium is transparent to infra red light with an exceedingly high refractive index of 6.3, the highest known. Tellurium is a p-type semiconductor and its conductivity increases slightly with exposure to light. It can be doped with silver, copper, gold, tin or other elements. Cadmium sulphide, CdS; cadmium selenide, CdSe; and cadmium telluride, CdTe, are type II/VI photoconductive semiconductors used in the detection of light. Tellurides are analogous to sulphides. Telluric bismuth is an inter-metallic compound, Bi3Te3. Tellurium, being a bigger atom than sulphur, can accommodate more oxygen atoms around it, so telluric acid is H6TeO6, and when heated yields the trioxide, TeO3. Salts of telluric acid are called tellurates. Salts of H2TeO4, the analogue of sulphuric acid, exist, but not the acid itself. Tellurium is used to tint glass. Tellurium is probably toxic, and if the dust is inhaled, 'tellurium breath', a garlic-like odour on the breath similar to selenium poisoning results, probably as a result of dimethyl tellurium, Te(CH3)2, forming. Tellurium is in-essential for life and is fairly toxic, but the low solubility of most of its compounds renders it relatively safe. Tellurium is obtained from the flue dusts produced in the processing of copper sulphide ores are used and from the anode slimes of electrolytic copper refineries; and from sylvanite, silver gold telluride, AgAuTe4 or from calaverite and krennerite, both gold tellurides, AuTe2. Tellurium is occasionally found native and in some rare minerals, like tellurite, orthorhombic tellurium dioxide, TeO2, and in tetradymite, bismuth tellurium sulphide, Bi2Te2S. Natural tellurium is slightly radioactive and consists of eight stable isotopes, three of which are mildly radioactive with enormously long halflives, the most abundant isotope is Te-130 at 35% which is subject to double beta decay with a halflife of 2500x1018 years, and 32% Te-128, another double beta decaying isotope with an even longer halflife of 50,000x1018 years, and 1% of a positron emitting isotope, Te-123 with a halflife of 13x1012 years. Altogether, another 25 radioactive isotopes are known, ranging from positron (and alpha) emitting Te-106 to electron (and neutron) emitting Te-138. With a halflife o 2500x1018 years, the activity of a one kilogram mass of pure tellurium-130 is, on average, 38×10-6becquerels per second, or one disintegration per 30,000 seconds. Measuring halflives of this period requires very careful shielding from background radiation. Claim to fame:
|
![]()