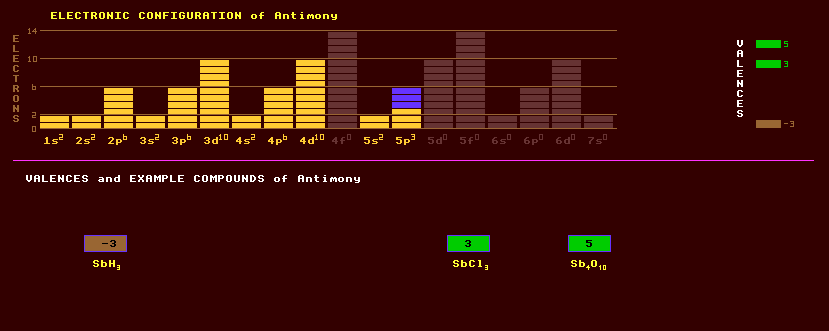

|
51 ANTIMONY Sb (Greek: anti + monos = not alone) Latin: Stibium
Antimony is an bluish-white extremely brittle metallic element with a metallic lustre and a flaky crystalline texture. It exists in two allotropic forms, the stable metallic form and the amorphous grey form. It is resistant to oxidation at room temperature, but burns brilliantly when heated producing fumes of the white oxide, Sb2O3. It is a poor conductor of heat and has a hardness of 3 to 3.5. Antimony is in the same group 15 as nitrogen, phosphorus, arsenic and bismuth. Antimony is alloyed with lead to improve its corrosion resistance and mechanical strength. Antimony is added to lead at between 4% and 12% for use as battery plates; and at 10% for use in lead shrapnel. Type metal is an alloy of lead, antimony and tin. Antimony black is finely divided antimony powder, and is added to plaster casts to give them a metallic look. Because of its volatility, antimony is found contaminating soils around incinerators and metal refineries. Antimony is added to semiconductor metals like germanium to act as a donor impurity. This makes the semiconductor an n-type semiconductor. Indium antimonide is an important semiconductor used as a photoconductor for detecting light in imaging devices. Antimony has no known biological function and many of its compounds are toxic. Tartar emetic, hydrated potassium antimonyl tartrate, is used as an emetic. The maximum recommended concentration of antimony dust in air is 0.5 mg/m3. That for stibine is a mere 0.1 ppm. Stibine, SbH3,has recently been implicated in baby cot deaths, where it was once wrongly thought that a fireproofing compound of antimony added to polyurethane and polystyrene mattresses reacted with urine to produce this toxic gas, which is 1000 times more toxic than carbon monoxide, and acts as an anti-cholinesterase, like organophosphorus pesticides and nerve gases. There also exists a soluble solid dihydride, Sb2H2. Antimony oxide, Sb2O3, is used as a white hiding pigment. Antimony is found in over a hundred minerals, sometimes as native antimony, but more frequently as the sulphide, antimonite (also known as stibnite or antimony glance), Sb2S3, a shiny black metallic looking acicular bunch of crystals which unusually can easily be bent without fracture as its crystal planes will slip easily over each other a certain distance before stopping. Antimony is also found as the antimonide of the heavy metals, like pyragyrite, Ag3SbS3; stephanite, Ag5SbS4; polybasite, (Ag,Cu)16Sb2S11; bournonite, PbCuSbS3; boulangerite, Pb5Sb4S11, looking like a jumbled bunch of embroidery pins; jamesonite, Pb4FeSb6S14, and tetrahedrite, Cu12Sb4S13. Berthierite, FeSb2S4 is an iron antimonide sulphide, miargyrite a silver antimony sulphide, Ag2Sb2S4. Senarmontite, Sb2O3, occurs as rare octahedral crystals in the oxidation zone of antimony bearing deposits, as does the richly faceted valentinite, of same chemical formula. Antimony is extracted from the sulphide by first roasting to the oxide, then reducing with salt and scrap iron or with carbon. Antimony has 29 isotopes ranging from antimony-188 to antimony-136. Two stable isotopes exist, antimony-121 and -123, existing in near equal proportions. Some radioactive isotopes are used as intense gamma and neutron sources. Claim to fame: Antimony is the only? known metal to expand on solidifying. This curiosity is used to great effect in making lead type, where the addition of a little antimony not only hardens the lead, but also makes it expand on solidifying thus creating higher definition casts.
|
![]()