
|
50 TIN Sn Stannum
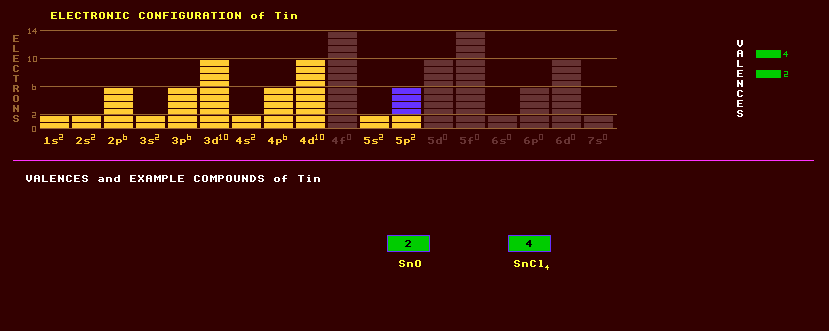
Tin is a soft, pliable, silvery white, ductile and malleable metal which exists in three allotropic forms: grey tin (alpha tin) crystallizing in the cubic form changes into white tin (beta) with a tetrahedral structure at 13.2 Celsius. When tin is cooled below 13ºC it gradually changes from white tin to the powdery grey tin, this process is called tin pest. Some say tin has a third form above 161 C called gamma tin. Tin is unaffected by air or water because of the formation of a thin protective oxide coating, but dissolved in acids or alkalis. Tin is used to plate steel in what are commonly called 'tin cans', where it prevents the corrosion of the steel. Pure tin, like indium, lets out a high-pitched 'cry' when bent due to the breaking of tiny crystals. Alloys of tin with lead have a low melting point and are used as solder; the 60/40 alloy (with more tin), which is used for electrical connections, has a lower melting point than the 40/60 alloy (with more lead) used in plumbing. Like all binary alloys, both mixtures have much lower melting points than either constituent alone. Copper at 1% is sometimes added to electrical solder to prevent the copper bit used in soldering irons slowly dissolving in the solder. Bronze is an alloy of tin and copper, harder than either constituent. Pewter is an alloy of tin and lead, once used for tankards, but has falling out of favour recently because of the toxicity of the lead. Bell metal, white metal (used for bearings), and phosphor bronze are other alloys containing tin. Tin-zinc and tin-nickel are non-corrodible metal finishes applied by simultaneous electrodeposition of the two metals. A tin niobium alloy, Nb3Sn, is superconductive at cryogenic temperatures and used for generating massive magnetic fields in particle accelerators and medical magnetic body scanners. Tin oxide, SnO2, is transparent yet conducts electricity, and is used as a conductive coating on glass in liquid crystal displays (LCDs). Tin chloride, SnCl2.H2O, is used as a reducing agent and a mordant in calico printing. Stannous fluoride is used in some fluoride toothpastes, although 'fluoride' is now known to dangerously weaken bones after prolonged ingestion. Organic compounds of tin are often toxic, tributyl tin, a powerful pesticide, is used as an anti-barnacle additive to yacht varnishes, but is creating great havoc in the marine environment to other forms of harbour life. The main ore of tin is cassiterite, tin oxide, SnO2, otherwise known as tinstone, which occurs as stubby brown or black prismatic, often twinned, crystals highly prized by collectors and often in association with copper, tungsten and molybdenum ores. Roasting cassiterite with charcoal at 1000 Celsius yields metallic tin. Tin pyrites, stannite, Cu2FeSnS4, is a sulphostannate of copper and iron usually occurring in tin-bearing veins. In Britain, tin was traditionally mined in Cornwall but is now imported. Tin is essential to humans, but the toxic intake is 2 grams. Tin has twelve stable isotopes, ranging from Sn-112 to Sn-124, the most abundant of which is Sn-120 at 33%. Claim to fame: Tin has the greatest number of stable isotopes, 10 in all. Another 22 radioactive isotopes are known, ranging from positron emitting tin-103 to electron emitting tin-134.
|
![]()