
|
49 INDIUM In (indigo line in spectrum)
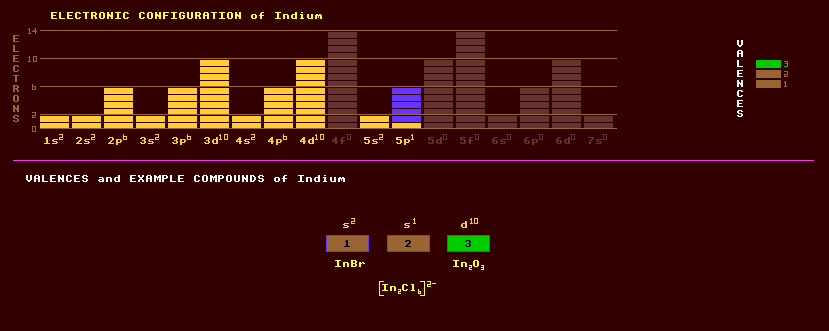
Indium is a soft silvery white metal with a brilliant lustre belonging to the same group as boron, aluminium and gallium, group 13. It is soft and marks paper like lead. Indium is stable in air and water, has very low solubility in water but dissolves in acids. The pure metal, like tin, emits a high-pitched 'cry' when bent. Like gallium indium wets glass making as good a mirror as silvered glass, but is tarnish resistant. Indium is fairly toxic and is not essential for life. Indium alloys are used to make high speed bearings. It makes low-melting point alloys, used in some solders and as the fusible tap in sprinklers etc. An alloy of 24% indium and 76% gallium is liquid at room temperature. Indium is a group III element (new group number 13), and alloyed with various elements of group V (new group number 15) such as (phosphorus, arsenic and antimony), it is forms an important class of semiconductors, the 3/5 semiconductors. Indium antimonide, InSb, is a semiconductor used as a photoconductor for detecting light in imaging devices and as a thermistor where its resistance is dependant on temperature. Indium gallium arsenide, InGaAs, and InGaAsP are used in photodiodes to detect light. The latter, in alternating stripes with indium phosphide, InP, forms a laser diode. Both indium oxide, InO, and indium tin oxide are transparent yet conductive, and are used as an electrode coating on some electro-optic cells and liquid crystal displays.
It has been proposed that indium-115 would make an excellent solar neutrino detector because it is about twenty times more sensitive to the low energy neutrinos emitted by the proton + proton fusion reaction than any of the other detectors based on chlorine-37, gallium-71 or Cerenkov detectors using water. The indium-115 absorbs an electron neutrino to become an excited state (isomer) of tin-115, which decays by gamma radiation into the ground state of tin-115. ( Indium has a body centred tetragonal crystalline form with two crystallographic axes. The thermal expansion of indium is unusual in that the length measured in the direction of the c-axes passes through a maximum around room temperature. Indium is strongly chalcophilic and is closely associated, in low concentrations, with many metal sulphide minerals including zinc ores, from which it is obtained. It is also extracted as a byproduct from iron, lead and coppers ores. Indium has a high absorption of slow neutrons and is readily activated. Indium is slightly radioactive and exists naturally as two isotopes, 4% indium-113, and 96% of the radioactive indium-115, which is subject to beta decay but with an enormously long half life of 439 Tera years. There are altogether 34 known isotopes of indium, ranging from indium-133 to indium-180. Claim to fame:
|
![]()