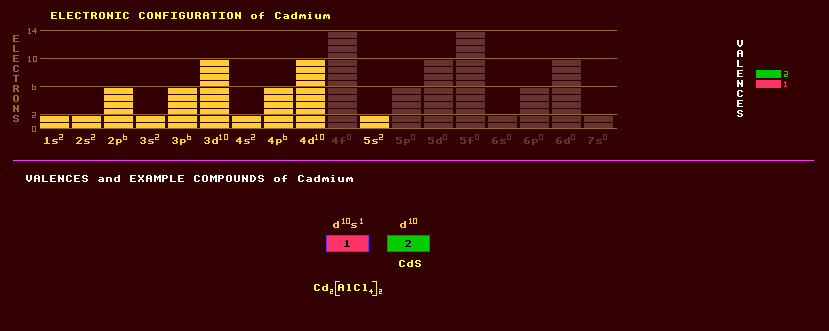

|
48 CADMIUM Cd (Latin: cadmia = calomine)
Cadmium is a soft, bluish-white transition metallic element that can be cut with a knife and is similar to zinc. Cadmium is used as the cathode in re-chargeable nickel-cadmium (Cd-NiO) batteries which produce an emf of 1.3 volts per cell, and in standard emf cells; and to cadmium plate steel and aluminium to give protection from corrosion. Added to copper at 1% it provides the strength needed for telephone and overhead power cables for trams and trains. Cadmium is a potent absorber of neutrons, used as gain control rods in nuclear reactors. Cadmium is a component of some of the lowest melting point alloys: Woods metal (4Bi,2Pb,Sn,Cd) melts at 71ºC, and Lipowitz alloy (15Bi,8Pb,4Sn,3Cd) at 60-65ºC. Cadmium red is a brilliant red but low opacity pigment used in paints comprising of a co-precipitated mixture of cadmium sulphide, cadmium selenide and barium sulphate. Cadmium lithopone is a reduced yellow co-precipitate of just cadmium sulphide and barium sulphate. Cadmium yellow is a pale yellow to light orange pigment of cadmium sulphide. Cadmium oxide, CdO, is transparent yet conductive, and is used as an electrode coating on some electro-optic cells. Cadmium sulphide, CdS, is a semiconductor used as a light sensitive resistor in photographic lightmeters, as its electrical resistance decreases with increasing light intensity, but has a slow response time. Cadmium telluride has a better response time. A mixture of yellow-emitting cadmium sulphide and blue-emitting zinc sulphide phosphors are used as the phosphorescent screen in black and white televisions. Cadmium is a poisonous metal producing neurological symptoms characteristic of heavy metal poisoning and is an environmental pollutant at many mineral mines. Cadmium is scavenged by and concentrated in marine organisms, which dwell in shallow waters, and is also a serious contaminant of sewage sludges, posing a disposal problem. In the body, cadmium competes with calcium, copper and zinc and other essential metals, interfering with their metabolism. Symptoms of poisoning include disfunction in lungs, kidneys and softening of the bones resulting in extreme pain in the joints. A diet rich in calcium or selenium helps protect against cadmium poisoning whereas a diet deficient in calcium or vitamin D exacerbates the toxic effects. Cadmium stimulates the production of the enzyme metallothionein, which forms a complex with cadmium (and with other heavy metals) safely sequestering it for later disposal from the body. However, it has limited capacity for the metal, which can accumulate in the body over the years if the influx of cadmium exceeds its capability. Dangerously poisonous and strangely yellowish fumes of cadmium vapour are emitted during the melting of cadmium and of silver solder, which contains cadmium. The principal ore of cadmium is greenockite, cadmium sulphide, CdS, which fluoresces orange yellow when rich in zinc. Cadmium is often found in association with zinc ores such as sphalerite, zinc sulphide, ZnS. Almost all cadmium is obtained as a by product from the processing of copper, zinc and lead ores. Cadmium exists as a mixture of seven stable isotopes of which Cd-114 is the most abundant at 28%. In addition 27 radioactive isotopes are known ranging from the positron emitting Cd-97 to the electron emitting Cd-130. Claim to fame:
|
![]()