
|
47 SILVER Ag Argentum
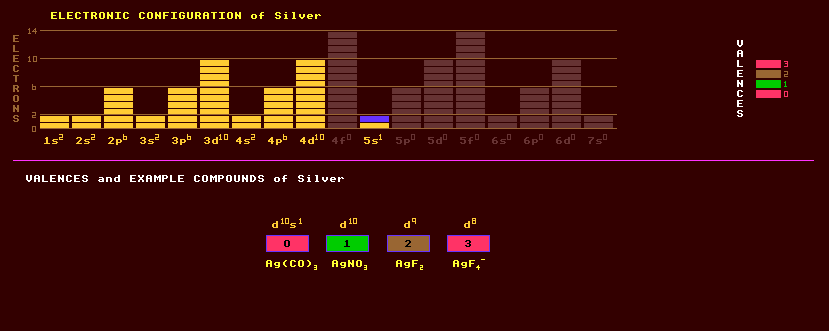
A pure white fairly soft, ductile, malleable, transition metal element belonging to the precious metals. Although not oxidised in air, metallic silver tarnishes with the formation of a patina of silver sulphide, being yellow, blue or black with increasing thickness. It can be beaten into silver foil which appears blue by transmitted light. Silver is a good conductor of electricity and heat, better even than copper, hence silver-plated copper wire. Silver is an expensive metal used in jewellery, cutlery, ornaments, silverware and silver plated copper. It finds use in small zinc-silver oxide batteries for watches and calculators. Also used for silvering glass mirrors. Silver steel is a bright drawn carbon steel alloy containing small amounts of silica, manganese, chromium and carbon. Silver exhibits a valency of +1 (common), and +2 or rarely +3. Some compounds of silver are sensitive to light, releasing metallic silver on exposure, like silver nitrate, AgNO3. Crystals of silver iodide, AgI, silver chloride, AgCl, and silver bromide, AgBr, are used in photographic emulsions, and account for half of the Worlds use of silver. The action of light in these crystals is greatly sensitised by the fortuitous formation of minute traces of silver sulphide in the gelatin emulsion base. Fulminating silver is not a fulminate at all but a nitride of silver, Ag3N, and is a sensitive explosive used in Christmas crackers. Silver acetylide, Ag2C2, made by bubbling acetylene gas through an ammoniacal solution of silver nitrate, is also explosively sensitive to touch when dry. Silver forms several oxides, argentic oxide, AgO; silver oxide (argentous oxide), Ag2O; silver suboxide, Ag4O; and a Ag2O2. Silver nitrate is the commonest salt, which in fused stick form is called lunar caustic, and was used in marking linen. Silver salts are poisonous to humans and microorganisms. At ultraviolet wavelengths, ultra-thin foil of silver is not only transparent, but exhibits a negative index of refraction. Argentite and acanthite, isometric and monoclinic forms of silver sulphide, Ag2S, are an important ore of silver and have a shiny lead-gray appearance. Argentite can be cut with a knife, which distinguishes it from galena or lead sulphide. Silver is often found in association with the lead ore galena, lead sulphide. Silver is sometimes found in nature as an amalgam with mercury, and rarely as native silver, when it usually takes on the appearance as intertwined distorted bundles of wires. Native gold is more common than native silver. Other minerals of silver are the scarlet red proustite, Ag3AsS3, prized by mineral collectors for its beauty; deep red pyragyrite, Ag3SbS3, but which turns grey on the surface when exposed to light due to the formation of a thin film of metallic silver; iron black stephanite, 5Ag2S.SbS3; polybasite (Ag,Cu)16Sb2S11; sylvanite, AuAgTe4; petzite, Ag3AuTe2; dyscrasite, Ag3Sb, a silvery hydrothermal mineral; and chlorargyrite, AgCl. Pyrargyrite, Ag3SbS3, is dark red and similar to proustite, but antimony substitutes for arsenic; it too turns dark on exposure to light. Hessite, silver telluride, Ag2Te, sometimes occurs in gold or silver bearing quartz veins.
Claim to fame: Silver has the highest electrical conductivity of any element and the lowest spectral emissivity of any metallic element (0.05 @ 0.65
|
![]()