
|
46 PALLADIUM Pd (Pallas - an asteroid)
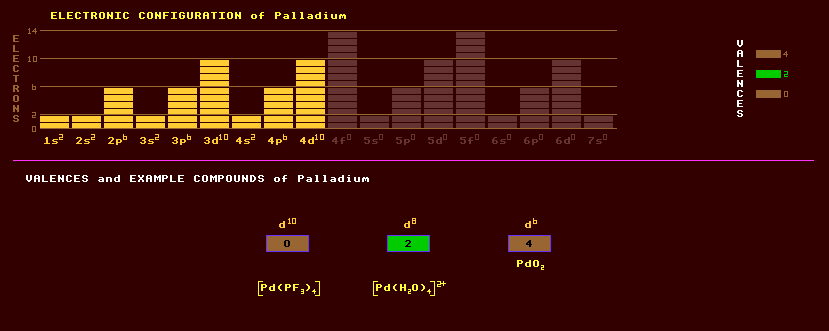
A silvery white, lustrous, malleable and ductile (when annealed) metallic element which resists corrosion. Cold working greatly increases its strength and hardness. Palladium resists corrosion, but dissolves in oxidising acids like nitric or sulphuric acids, and fused alkalis. It belongs to the platinum group metals and has the lowest melting point and least density of this group. Palladium is frequently alloyed with iridium and platinum and used in jewellery. White gold is gold alloyed with palladium, which decolourises it. Like gold, palladium can be beaten into thin foil only 4 millionths of an inch thick and is used as the delicate mainspring in wrist watches. Palladium is used to make good electrical contacts in electrical relays, and in making surgical instruments and in dentistry. Generally, palladium is non-toxic. Palladium is used as a catalyst in hydrogenation and de-hydrogenation in the petrochemical industry. Palladinized asbestos, a catalyst, is made by saturating asbestos with a palladium compound, then subsequently decomposing it to leave finely divided palladium dispersed throughout the asbestos. Palladious iodine, PdI2, is used to distinguish iodine from other halogens. Palladium is capable of absorbing up to 900 times its own volume of hydrogen gas, and when it does, its own volume increases by 10%, possibly forming Pd2H. Palladium is a rare and expensive metal whose price momentarily increased in 1986 when it was used as an electrode in an electrolytic cell containing lithium deuteride as electrolyte in experiments into cold fusion, where it was erroneously thought to help hydrogen (deuterium) to undergo nuclear fusion producing helium. A porous palladium block is used as an extremely fine method of controlling the entry of gas into a vacuum. The palladium leak, as it is called, is controlled by altering the temperature of the palladium block, and offers superior control to a needle valve. Hydrogen will readily diffuse through heated palladium, providing a means of purifying the gas. Native palladium is found mostly as grains in association with placer deposits of other platinum group metals. It has a very low abundance in the crust or Earth as a whole. Palladium is also extracted commercially as a by-product of copper, nickel and zinc refining. Palladium exists as a mixture of six stable isotopes, the most abundant of which is palladium-106 at 27% abundance, followed closely by Pd-108 at 26% and Pd-105 at 22%. The other stable isotopes are Pd-110 and Pd-104 at 11% and with just 1% Pd-102. In addition, twenty radioactive isotopes are known, ranging from the positron emitting Pd-94 to the electron emitting Pd-119. Claim to fame:
|
![]()