
|
45 RHODIUM Rh (Greek: rhodon = rose)
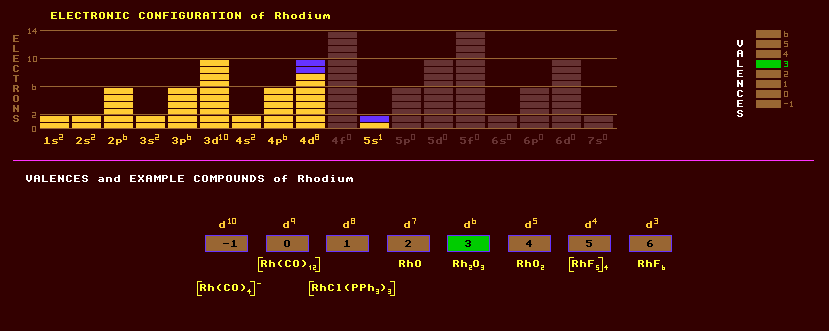
A rare, noble, hard, silvery white metal resembling platinum which is unaffected by air up to 602 Celsius when it slowly oxidises to rhodium sesquioxide, Rh2O3. At still higher temperatures, it reverts back to the metal. Rhodium belongs to the noble platinum group of metals. Rhodium is inert in acids, but is attacked by fused alkalis. Rhodium has a higher melting point than platinum, and is alloyed with platinum or palladium to harden them, when they are used as die bushes for glass fibre production, aircraft spark plug electrodes and laboratory crucibles. Alloyed with platinum, it is used in platinum rhodium/platinum thermocouples for measuring high temperatures. Also used for plating silver to prevent tarnishing. Rhodium finds use as a catalyst, especially catalytic converters in car exhaust systems to prevent the emissions of noxious gases, although when starting from cold, the exhaust then emits the characteristic smell of hydrogen sulphide. Which is worse: dirty exhausts, or farting cars? Rhodiums' low electrical resistivity finds use in electrical contacts, where its high resistance to corrosion is exploited. Plated rhodium is hard and has a high reflectance of light and is used in optical instruments. Rhodium occurs as the native metal in association with other noble metals, and also in trace amounts in a nickel ore, copper nickel sulphide. Rhodium exhibits valences from -1 to +6. The following oxides are known: Rh2O, RhO, Rh2O3 and RhO2·2H2O. Rhodium has four known fluorides, RhF3, RhF4, (RhF5)4, and RhF6. Salts of rhodium have a rosy red colour, hence the name. Rhodium exists entirely as one stable isotope, rhodium-103. A further twenty three radioactive isotopes are known, ranging from the positron emitting Rh-94 to the electron emitting Rh-117. Rhodium-104 can decay by either of the two routes, inverse beta decay, or beta decay. Claim to fame:
|
![]()