
|
44 RUTHENIUM Ru (Latin: Ruthenia = Russia)
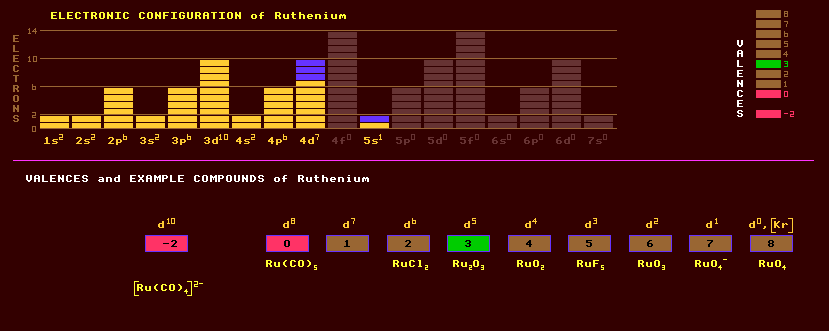
A rare, hard, brittle, lustrous silvery white metallic element which belongs to the platinum family with which it occurs with other platinum metals in the ore osmiridium, a naturally occurring alloy of osmium and iridium. Ruthenium is unaffected by air, water and acids, but dissolves in molten alkali. It oxidises in air at 800 Celsius and is also attacked by halogens. It also occurs in the nickel iron sulphide, pentlandite, (Fe,Ni)9S8 in Ontario, and in pyroxenite deposits (an ultramafic intrusive igneous rock) in South Africa. Laurite is ruthenium sulphide, RuS2. It is used to harden platinum and palladium for making electrical contacts in relays etc. The corrosion resistance of titanium is increased a hundred fold just by adding 0.1% ruthenium. It is used in electrodes for the production of chlorine by electrolysis. An alloy of ruthenium and molybdenum is superconductive below 10.6 Kelvin. Ruthenium finds most use as a catalyst. Ruthenium exhibits a wide range of valences from -II to VII by gradually filling up the d-shell. Ruthenium has four oxides, the sesquioxide Ru2O3, the dioxide RuO2, the trioxide RuO3 and the highly toxic tetroxide RuO4 an analogue of the highly toxic osmium tetroxide, OsO6; and the following fluorides RuF2, RuF3, RuF4, RuF5 and RuF6. The compounds show a marked resemblance to those of osmium. Apart from the toxic tetroxide, ruthenium is harmless but in-essential for life. Ruthenium occurs naturally as seven stable isotopes, 5% Ru-96, 2% Ru-98, roughly equal proportions of Ru-99, Ru-100, Ru-101 and Ru-104 at about 13%, but the most abundant isotope is Ru-102 at 32%. A further sixteen radioactive isotopes are known, ranging from the positron emitting ruthenium-91 to the electron emitting ruthenium-113. Radioactive ruthenium isotopes are one of the many products of the fission of the heavier elements and produced within nuclear reactors and atomic explosions. The radioactive isotope, ruthenium-106, with a halflife of 372 days, has been found in the Irish sea, a consequence of Sellafield discharges, both accidental and 'lawful', where it is taken up by the edible seaweed Porphyra, which was made into laver bread in South Wales and eaten, contaminating humans. This is one of the most notorious cases of radioactive fission products turning up in the human food chain. The Welsh now use other sources of Porphyra in the making of laverbread. Claim to fame:
|
![]()