
|
43 TECHNETIUM Tc (Greek: technikos = artificial)
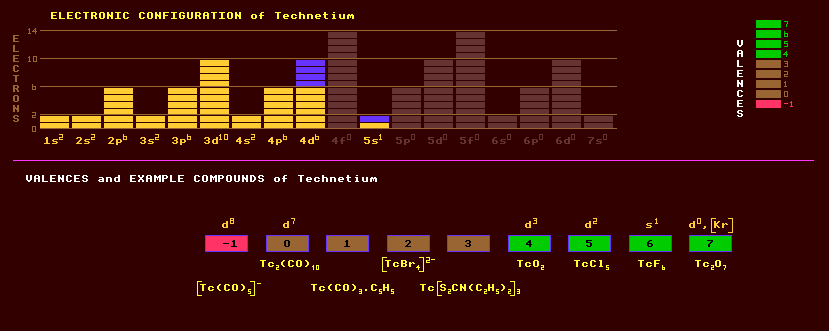
Technetium does not occur naturally because all its isotopes are radioactive and if any was made in supernova stars, then it has long since been transmuted into other elements by radioactive decay. It is the first element in the periodic table to be wholly radioactive, with no stable isotopes. It has an odd number of protons in the nucleus. Technetium is the first artificially produced element. It was first produced artificially in 1937 by bombarding molybdenum with deuterons and neutrons within a cyclotron. Technetium is also found in the fission products of uranium, and also in the spectra of S-type, M-type and N-type stars, where it is synthesized by nuclear reactions. The most common isotope, technetium-99, has a half-life of 213,000 years. Named from the Greek, technetos, meaning artificial. Technetium is a silvery grey metal but usually obtained as a grey powder. It resists oxidation, tarnishing only slowly in moist air, but burns in oxygen, and dissolves in nitric and sulphuric acids. As the compound, technetium pertechnate, KTcO4 dissolved in water at a concentration of only 5ppm, it is a remarkable corrosion inhibitor for steel, but its high specific radioactivity prohibits its general use. Like its chemical analogue, manganese, technetium exhibits a large number of valences: -1, 0, +1, +2, +3, +4, +5, +6 and +7, the most commonly expressed being +4, +5 and +7. With so many possible oxidation states at its' disposal, technetium makes a good catalyst. Chemically, technetium behaves more like its heavier rhenium homologue than its lighter manganese homologue. It crystallizes in the hexagonal close-packed arrangement like rhenium and not in the cubic system of manganese. Like manganese, many of its' compounds are highly coloured. Amongst the compounds that have been prepared are a black-brown technetium dioxide, TcO2; the red technetium trioxide, TcO3; and the light-yellow technetium oxide Tc2O7, which is the oxide produced when technetium metal is burnt in oxygen. The fluorides include a bright red technetium tetrachloride, TcCl4, pentachloride, TcCl5, and a golden-yellow hexafluoride, TcF6 and two oxychlorides, TcO3Cl and the blue TcOCl4. A carbonyl compound Tc2(CO)10 and a carbonyl complex, [Tc(CO)5]- are known. Other complexes are [TcO3]2-, [TcO3]- in aqueous solution, [TcO4]- again in aqueous solution. A nine-co-ordinated [TcH9]2- species is also known Technetium-99 has a long halflife of 213,000 years, and is one of the many waste products formed in the fission of uranium within nuclear reactors, and can be extracted from the wastes during re-processing. A 100 MW nuclear reactor produces about 2.6 grams of technetium-99 per day. It is thus now more available than many other rare elements. The levels of technetium-99 found in the Irish Sea, a consequence of both legal and 'accidental' discharges from Sellafield, have increased by forty-fold since 1993. The levels found in lobsters and other shellfish caught in that area are now causing concern. Technetium-99m, the excited metastable state of technetium-99 which emits medium energy gamma rays of 140keV energy when decaying back to technetium-99, has a very short halflife, 6 hours, making it useful as a medical tracer as it emits no alpha or beta particles. As such, it has largely replaced iodine-131 in this role. The short half-life of six hours ensures a low radiation dose to the patient and activities up to 1GBq can be safely administered. It used to be prepared by irradiating technetium-99 in a particle accelerator but is now generated almost continuously off the shelf from a molybdenum-99 pre-cursor which has a half-life of 66 hours. The molybdenum-99 beta decays into the metastable Tc-99m. It is then quickly injected into the body where it decays, emitting gamma rays. A gamma ray detector scans the body tissues. After its decay, the much longer-lived technetium-99 is safely excreted. A great many highly-organ-specific radiopharmaceuticals based on compounds containing technetium-99m are now used for imaging various organs and bio-chemical systems. Like rhenium, technetium can also exhibit a quadruple bond between two atoms of itself in complexes. Technetium-99 is a radiological contamination hazard. Twenty five isotopes are known, ranging from Tc-88 to Tc-113. The longest lived isotope of technetium is technetium-98 with a halflife of 4.2 Million years decaying by beta decay into the stable ruthenium-98. Technetium is an excellent superconductor with a high superconducting transition temperature of 8.2 Kelvin.
Claim to fame: The first element in the periodic table to have no stable isotopes.
|
![]()