
|
42 MOLYBDENUM Mo (Greek: molbdos = lead)
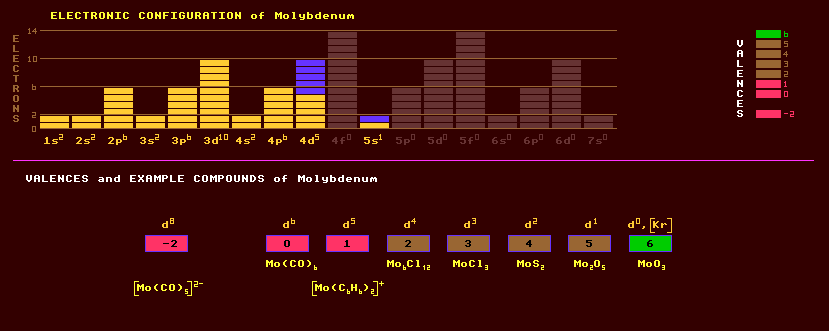
A lustrous, silvery, and very hard high melting point transition metal whose physical properties are similar to iron, but whose chemical properties are similar to a non-metal. It is usually obtained as a grey powder. Molybdenum wire is more ductile than tungsten wire. Molybdenum oxidises at high temperatures. Molybdenum wire is used to support filaments in electric lamps, for grids, screens and filaments in radio valves, as the electrodes in mercury arc lamps, and as resistance wire in electric resistance furnaces. Molybdenum is used in making the magnetic alloy permalloy and the very hard stellite alloys used for cutting tools which contain chromium, cobalt, tungsten and molybdenum in various proportions. It is also used in certain alloys with nickel in Hastelloys, which are heat and chemical corrosion resistant. Molybdenum exhibits a wide range of valences from -2 to +6, with only -1 being unknown. Molybdenum forms molybdenum(VI)oxide, MoO3, which with water forms molybdenic acid, H2MoO4. A dioxide, MoO2 and pentoxide, Mo2O5, are also known. Halides are Mo6Cl22, [Mo2Cl8]4-, MoCl3, MoCl4, and MoCl5 but only the fluoride MoF6, can elicit the +6 valency. Molybdenum is an essential trace mineral for plants, and is the active part in many enzymes. Nitrogenase is an enzyme containing molybdenum found in the bacteria inhabiting root nodules on some plants, the legumes, which has the ability to fix atmospheric nitrogen and turn it into a form that is usable by the plants. Clover and peas are are amongst the very few plants which can accomplish this feat of producing their own fertilizer. Molybdenosis, or teart, is a disease affecting cattle and sheep causing chronic diarrhoea when high levels of molybdenum are present in the soil. Molybdenum is used as the catalyst to de-sulphurise oil. Strictly speaking, molybdite is molybdenum oxide, MoO3, but so called molybdite is actually ferrimolybdite, a hydrous ferric molybdate, and is also known as molybdic ochre. Molybdenite, molybdenum disulphide, MoS2, which occurs as lustrous lead-grey crystals in association with granite and with copper ores, is the most common ore of molybdenum and crystallizes in the hexagonal system. Molybdenum disulphide is used as a dry lubricant resistant to high temperatures. The coefficient of friction of molybdenum disulphide at 0.02 is half that of teflon, but the new record holder is the ultra-hard evaporated NFC or near friction-less carbon which has a coefficient of friction of just 0.001. Minor ores are wulfenite, lead molybdate, PbMoO4, occurring as orangish crystals much sought after by collectors; and powellite, Ca(MoW)O4. Lead molybdate and calcium molybdate, CaMoO4, are insoluble in water. Molybdenum is also recovered as a by-product of copper and tungsten mining. Molybdenum exists as seven stable isotopes, the most abundant of which is molybdenum-98 at 24% abundance, the others Mo-92, Mo-94, Mo-95, Mo-96, Mo-97 and Mo-100 comprise the remainder varying from 9% to 16% abundance. Sixteen radioactive isotopes are known, ranging from the positron emitting Mo-87 to the electron emitting Mo-109. Claim to fame:
|
![]()