|
41 NIOBIUM Nb (Greek: Niobe - daughter of Tantalus) [formerly Columbium, Cb]
Niobium is a rare, shiny, silvery metal that is soft and ductile when pure. It resists corrosion due to the formation of a bluish protective oxide film on the surface, and is unaffected by fused alkalis, but is attacked by hot concentrated acids. Niobium starts to oxidise at temperatures above 200 Celsius.
Niobium is used in stainless steels and in arc welding rods for stabilised stainless steels, and in other high temperature alloys for gas turbines and nuclear reactors owing to the strength of its alloys at temperatures above 1200 Celsius. Alloyed with tin, it forms an important superconductor, niobium tin, Nb3Sn, which has the relatively high superconducting critical temperature of 18.45 Kelvin and a critical field at 4 Kelvin of 26 Tesla and is used in cryogenic electromagnets. Superconducting magnets have also been made with a niobium zirconium alloy wire. A niobium aluminium alloy is also superconducting. Of the intermetallic superconductors, niobium germanium, Nb3Ge, has the highest superconducting critical temperature of 23.2 Kelvin.
Niobium itself is superconducting below 9.2 Kelvin, indeed, it has the highest superconducting transition temperature of any element. Superconductors have very low acoustic losses for sound waves (phonons) travelling through them, superconducting niobium especially so. This makes it ideal as a gravitational wave detector, where a huge mass (the bigger the better) of superconducting niobium is carefully isolated from external vibrations in order to detect the extremely small distortions in space caused by the passage of a gravitational wave, perhaps caused by the energetic merging of two neutron stars or black holes. (Spherically symmetric gravitational collapses generate no gravitational waves, only asymmetric ones do. This is because the graviton, a spin-2 particle, is quadrupole in nature and generates tidal forces which causes an object to expand in one direction whilst contracting in the other orthogonal direction). The passage of a gravitational wave would cause an object to suddenly change shape and leave it vibrating. The low acoustic loss in niobium gives it an enormous Q-gain (over 10,000,000) enabling it to ring like a bell for days after being 'struck', greatly enhancing its sensitivity. So far, despite enormous effort, no gravitational wave has yet been unequivocally identified. This is because, despite the enormous power released as gravitational waves by the coalescence of two black holes, the deformation of space caused by the radiating gravitational wave is extremely small precisely because space is so very stiff, being 1027 times stiffer than diamond, the hardest physical substance known.
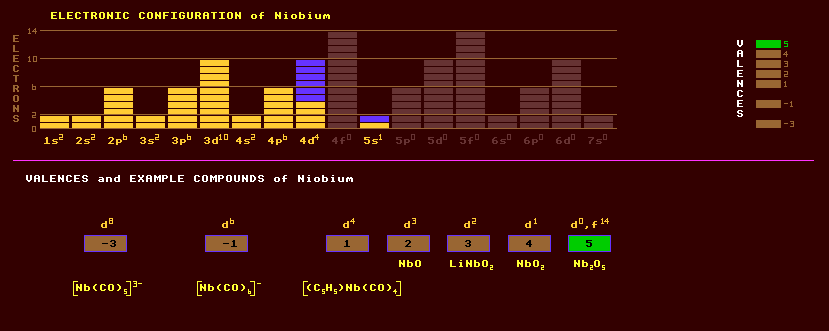
Niobium exhibits a range of different valences between -3 to +5. The following oxides are known: NbO, NbO2, Nb2O5; the fluorides NbF3, NbF4 and NbF5; and the oxyhalide NbOCl3.
Many niobates (and tantalates and titanates and some zirconates and hafnates) crystallize in the anisotropic perovskite structure and generally exhibit a range of active phenomena, many are either ferroelectric or anti-ferroelectric. Thus lithium niobate, LiNbO3, is ferroelectric, that is, below a critical temperature, 1207 Celsius in this case, the positive ions in the crystal structure spontaneously displace themselves with respect to the negative ions giving rise to a spontaneous electrical polarization across certain crystal faces, analogous to the spontaneous magnetisation of ferromagnetic substances below the Curie temperature. The critical temperature has the same name, Curie temperature, though it applies to different phenomena. This ferroelectric transition is really a phase change transition as the crystal changes from one structure to another. Analogous with ferromagnetic substances, both the electric susceptibility and dielectric constant become infinite below the Curie temperature, and a spontaneous electrical polarisation sets into the crystal in domains, but because of the random orientation of these domains, the net polarisation of the crystal is zero. Like ferromagnetic substances, a permanent (remanent) electrical polarisation can be set in the crystal by applying a large electric field across it whilst cooling the crystal down through the Curie temperature which aligns the domains into one particular orientation. Lithium niobate can be given a permanent electrical polarisation by the application of an external electric field when above 1127 Celsius; the saturation polarisation is very high at 50 microCoulombs/cm2. The spontaneous electric field exhibits hysteresis just like the spontaneous magnetic field in ferromagnetic substances.
Ferroelectric substances have unusually high and extremely temperature dependant properties which include the dielectric constant, the pyroelectric effect, the piezoelectric effect, and the electro-optic effect. Ferroelectric crystals are used as pyroelectric detectors in infrared (heat) sensing intruder alarms where an rise in temperature of the crystal generates a voltage (strictly speaking a charge) across two of its faces. Most ferroelectric crystals are also electro-optic, that is able to rotate the plane of polarization of light to an extent depending upon the magnitude of an applied voltage across the crystal. Hence lithium niobate is also used as an electro-optic modulator within a Pockels cell for fibre optic communications. Transparent ferroelectric crystals are useful for optical frequency doubling because of their highly non-linear optical properties at high light intensities typical of lasers. Ferroelectric materials are also useful in electret microphones, where the ferroelectric crystal provides a permanent polarisation voltage necessary for the condenser microphone to work.
All ferroelectric crystals are also piezoelectric (that is they generate a charge which produces a voltage change across opposite faces when a force is applied) but the converse is not true: quartz is piezoelectric only, whereas barium titanate is both ferroelectric and piezoelectric. Above the Curie temperature ferroelectric properties vanish but piezoelectric properties remain, the crystal becomes paraelectric (analogous to paramagnetic) accompanied by a large decrease in dielectric constant. The magnitude of the dielectric constant, pyroelectric coefficient and specific heat of ferroelectrics rises to a large peak just below the Curie temperature and thereafter suffer a large decrease (directly to zero in the case of the pyroelectric coefficient).
Niobium is lithophile commonly found in association with tantalum, especially in columbite, (Fe,Mn)(Nb,Ta)2O6, a collective name for a complete isomorphic series between niobite (Fe,Mn)Nb2O6 and tantalite, (Fe,Mn)Ta2O6, which are all hard and very heavy, metallic-black iron manganese niobium tantalates. Large quantities of niobium have been found associated with carbonatites (carbon-like silica rocks) as a constituent of Pyrochlore, (Ca,Na)2(Nb,Ti,Ta)2O6(OH,F,O). Niobium can also occur in Euxenite, (Y,Er,Ce,La,U)(Nb,Ti,Ta)2(O,OH)6, and polycrase, (Y,Er,Ce,La,U)(Ti,Nb,Ta)(O,OH)6, which are commonly metamict (altered by radiation). Also in Stibiocolumbite, SbNbO4; and fergusonite, (Y,Er)(Nb,Ta)O4. The minerals of niobium are all highly insoluble in water.
Niobium has just one stable isotope, Niobium-93. A further 21 radioactive isotopes are known, ranging from the positron emitting niobium-84 to the electron emitting niobium-106. Niobium has a low thermal neutron capture cross section.
Claim to fame: Niobium has the highest superconducting critical temperature (9.2K) and highest superconducting critical magnetic field (206 mTesla) of any element.
|

![]()
![]()