
|
4 BERYLLIUM Be (Greek: beryllos = beryl)
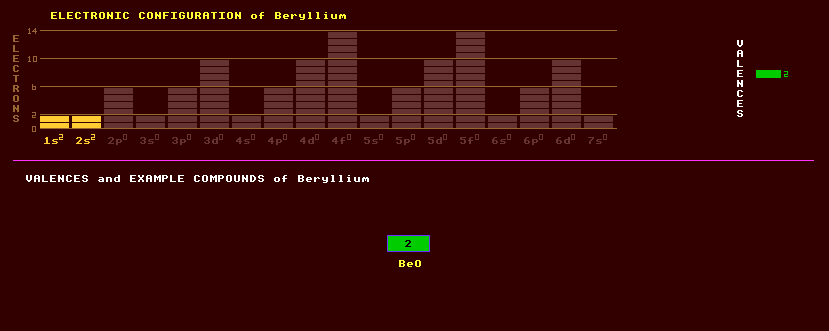
A steely in-corrodible white relatively soft alkali metal which is un-reactive with water. Beryllium is a rare element. Alloyed with copper forms beryllium copper which is used for springs. It was once called glucinium due to the sweetness of some of its compounds, but tasting beryllium compounds is not recommended due to their extreme toxicity. Because it is transparent to X-rays has found use for windows in X-ray tubes and was also used as a luminescent powder in fluorescent tubes until found poisonous. The metal can be evaporated onto glass, forming a mirror for ultraviolet light. As a slight alloy with nickel, it has the highest coefficient for secondary electron emission, 12.3. Alpha-particles projected into beryllium make it a useful source of neutrons, from which they were discovered by Chadwick in 1932. The oxide, beryllia, is a good reflector of neutrons and is also used in thermoluminescent dating, in power transistors to electrically insulate but thermally conduct away heat, and in brake pads due to its heat-resistant properties, but is unfortunately highly toxic, being easily absorbed through the skin. Minute quantities of beryllium oxide dust are produced in the combustion of coal and oil. Found in nature as the gemstone mineral beryl, beryllium aluminium silicate, Be3Al2Si6O18, which is colourless and highly insoluble in water. It is also found in coloured varieties (deep crimson: the rare bixbite; red: red-beryl; green: emerald; blue: aquamarine; deep blue: maxaxite; pink to violet: morganite and verobevite; yellow: golden beryl; light yellow green: heliodore; and a colourless goshenite) which are its principal ores. Emeralds are a much more valuable gemstone than are aquamarines. Emerald owes its green colour to traces of chromium oxide, morganite its pink to caesium; maxaxite its deep blue to boron and red beryl to manganese. Beryllium is obtained by the reduction of beryl and like minerals or by the electrolysis of beryllium chloride. Chrysoberyl, Al2BeO4, is another beryllium ore and used as a gemstone when finely coloured. Chrysoberyl is the next hardest mineral to diamond and corundum. Alexandrite is a gemstone chrysoberyl that is deep green in daylight but mauve in tungsten lighting; it is thus said to be an emerald by day and an amethyst by night. In addition to the change in colour with lighting, alexandrite is strongly pleochroic, appearing red, orange-yellow and green in different crystal directions. Bertrandite, hydrous beryllium silicate, Be4Si2O7(OH)2, is a mineral of interest only to collectors. Beryllium is also present in milarite, K2Ca4Be4Al2Si24O60·H2O, in euclase, BeAlSiO4(OH) and in gadolinite, Be2FeY2Si2O10, an ore of yttrium. Beryllium carbide, Be2C, liberates methane gas, CH4, on hydrolysis with water. Beryllium consists of just one stable isotope, beryllium-9. Just a trace of the beta decaying radioactive isotope beryllium-10 exists on Earth, has a halflife of 1.6 million years, and is produced in the upper atmosphere by cosmic ray bombardment of dust. The radioactive isotope beryllium-8 has such a short halflife of just 200 atto seconds, that it is responsible for the longevity of the sun. Two helium-4 atoms, when fused together by nuclear fusion, would produce beryllium-8, but this has a fantastically short half life and doesn't last very long. So there is only a very small chance that during this time another helium-4 nucleus will collide and fuse with it to produce the stable carbon-12. Beryllium-8 is the bottleneck that prevents the suns reactions from proceeding at explosive pace. It appears that almost all of the Earths' boron (and lithium & beryllium) was created by extra-terrestrial cosmic ray bombardment of heavier nuclei in the interstellar medium causing their fragmentation in spallation reactions before the formation of the solar system. Claim to fame: beryllium has the highest velocity of sound transmission, 12890m/s.
|
![]()