
|
39 YTTRIUM Y (Ytterby, a town in Sweden)
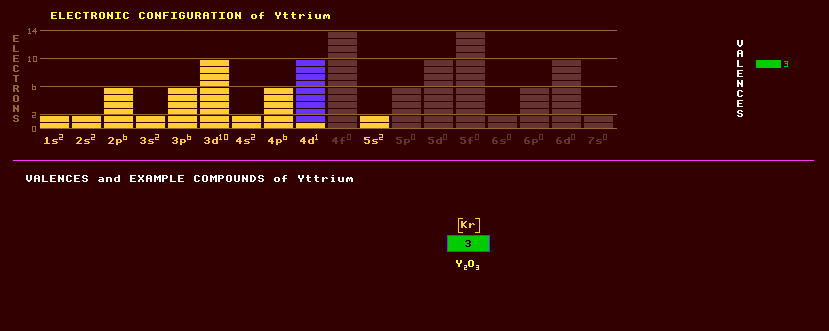
A soft, silvery white metallic element usually classed with the rare earths because if it chemical resemblance to them. It is stable in air due to the formation of a thin oxide layer, but reacts with water to produce hydrogen gas and the sesquioxide, Y2O3. Burns readily in air if heated to 400 Celsius. Small amounts of yttrium can be added to chromium, molybdenum, zirconium or titanium to reduce the grain size of the metals, and to aluminium or magnesium alloys to increase their strength. It is used as a deoxidiser for vanadium and other non-ferrous metals. Yttrium is used in X-ray filters, and in superconductors and superalloy, a high temperature alloy based on iron, chromium, cobalt, manganese and or nickel, which can withstand temperatures up to 1000 Celsius. YAG crystals, yttrium aluminium garnet, Y3Al5O12, are used as microwave amplifiers (masers) and because it it is very hard (hardness 8.5) as a simulated diamond in jewellery, as is GGG, a gadolinium gallium garnet. YIG, yttrium iron garnet, Y3Fe5O12, is used as an exceptionally efficient ultrasonic transmitter and receiver. A yttrium gadolinium garnet is also used. Yttrium garnets have interesting ferroelectric and magnetic properties. A mixture of yttrium vanadate, YVO4 and europium; or a mixture of yttrium oxide, Y2O3 and europium is used as a red light emitting phosphor in colour television screens. Yttrium has a valency of 3 only. Thus YF3, Y(CO3)3 and other compounds are able to form. Yttrium is recovered commercially from monazite sand, (Ce,La,Nd,Th)PO4 which can be metamict (altered by irradiation) if it contains thorium, and which contains about 3% yttrium mixed up with other rare earths; and also from bastnasite which contains about 0.2% yttrium. Gadolinite, Be2FeY2Si2O10, is also an ore of yttrium and other rare earths. Yttrium occurs in the blue-violet earthy mineral yttrocerite, a cerium ore, consisting essentially of cerium fluoride and also in the orthorhombic mineral yttrotantallite, consisting of yttrium and tantalum, though niobium, cerium, uranium, iron or calcium can also be present in varying amounts. Xenotime is a yttrium phosphate, YPO4. Moon rocks have a relatively high yttrium content. Yttrium metal is now made by reduction of yttrium fluoride with calcium metal. Yttrium exists as just one stable isotope, yttrium-89. A further 22 radioactive isotopes are known, ranging from the positron emitting Y-80 to the electron emitting Y-102. Yttrium-89 has a low cross section for nuclear capture. Yttrium-9, a beta emitting radioactive isotope, is now to be found on Earth, existing in secular equilibrium with strontium-90, its source, which is a product of nuclear fallout from atmospheric testing of nuclear weapons and accidental discharges from nuclear power stations. Claim to fame: At 1 millibarn, Yttrium has the lowest thermal neutron capture cross sectional area for a solid element.
|
![]()