
|
38 STRONTIUM Sr (Strontian - Argyll, Scotland)
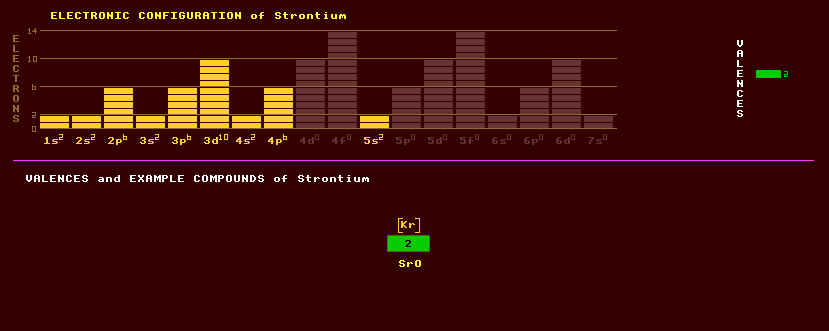
Strontium is a soft, silvery white metallic element first discovered in Strontianite, named after a place called Strontian in Scotland where it was found. Strontium is in the same group, group 2, as beryllium, magnesium, calcium, itself, barium and radium and is an alkali earth metal. Freshly cut strontium is silvery, but rapidly tarnishes in air with the formation of a protective yellowish oxide film on the surface, but will burn in air. Finely divided strontium will spontaneously ignite. Strontium reacts with water more strongly than does calcium and it should be kept under paraffin. Three allotropic forms of strontium are known: the stable face centred cubic alpha strontium becomes hexagonal close packed beta strontium above a temperature of 25 Celsius, which becomes body centred cubic gamma-strontium above 540 Celsius. Strontium is used in special glasses for TVs and VDUs. Strontium salts impart a beautiful crimson colour to flames, one of the tests for strontium. Strontium nitrate, SrNO3, is used as an oxidant in fireworks and in emergency flares to produce crimson coloured light. Strontium hydroxide, SrOH, has been used in sugar refining. Strontium titanate has an extremely high refractive index, and an optical dispersion (change in refractive index with wavelength) higher than that of diamond, and has been used as a gemstone but it is very soft, and does not occur naturally. Strontium occurs in the mineral celestite, strontium sulphate, SrSO4, which is commonly blue but can be colourless yellow or reddish; and strontianite, a colourless carbonate sometimes pale green or grey to brown, SrCO3; and also in mineral springs. Some microscopic marine organisms construct their skeleton with strontium sulphate, possibly accounting for deposits of celestite. Strontium Ruthenate, Sr2RuO4, is so far (2004) the only superconductor whereby the electrons pair up not with anti-parallel spins, but with parallel spins, something that has been known to be theoretically possible for some time. Such a superconductor is called a spin-1 bosonic superconductor (rather than a spin-0 bosonic superconductor) and should not have a superconducting critical magnetic field. See Superconductivity [POP]. It is superconducting at temperatures below 1.5 Kelvin. spin-1 states are known only in this superconductor, in superfluid liquid helium-3, and in the neutrons in extremely hot neutron stars. Of the elements that seem to be in-essential for humans, it has the highest human abundance. Strontium occurs as a mixture of four isotopes, 83% of which is strontium-88, 10% strontium-86, 7% strontium-87 and just 0.6% strontium-84. Altogether, 22 radioactive isotopes of strontium are known, ranging from the positron emitting strontium-77 to the beta decaying strontium-102. Strontium-90, with a halflife of 27 years, has achieved notoriety through being a radioactive product of uranium and plutonium fission within nuclear bombs and power stations. Cows eating grass from grassland contaminated with strontium-90 fallout pass the strontium into their milk. Through its chemical resemblance to calcium, the strontium-90 contaminated milk becomes incorporated into bones, where it can cause leukaemia. The strontium-90 emits damaging electrons but no gamma rays. A strontium unit is used to measure strontium-90 in calcium and is equal to 10-22 curies per gram. Strontium-90 is one of the best long-lived high-energy beta emitters for use as remote nuclear-electric power source known, and is used in navigation buoys, isolated weather monitoring stations and space satellites. Strontium dating uses the radioactive decay of rubidium-87 (with a halflife of 5x1010 years) into the stable strontium-87 to measure the geological age of rocks (The relative abundance of the two isotopes gives a 'strontium age'). Claim to fame:
|
![]()