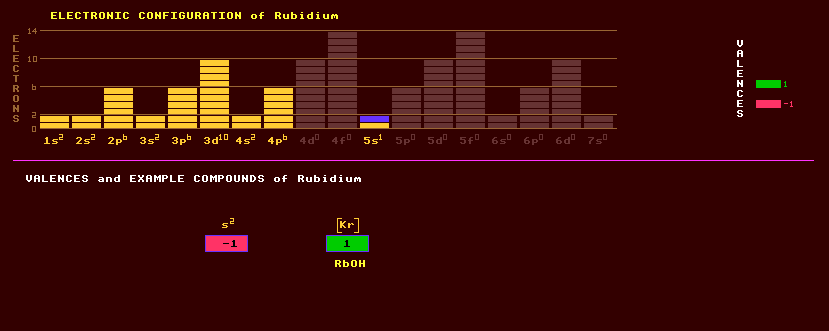

|
37 RUBIDIUM Rb (Latin: rubidius = deepest red)
Rubidium is a very soft silvery white and very rare alkali metal with a silvery white lustre when cut, traces of which are widely distributed, particularly in mineral waters. Rubidium reacts violently with water forming the alkaline hydroxide, RbOH and setting alight to the liberated hydrogen gas, and ignites in air to form the monoxide, Rb2O. Rubidium belongs to the same group 1 alkali metals as does lithium, sodium, potasium, itself and caesium. Rubidium can be liquid at hot room temperatures, as its melting point is only 39 Celsius. Rubidium is used in highly accurate atomic rubidium clocks. As with other alkali metals, its' compounds are generally water soluble and it forms amalgams with mercury and alloys with gold, caesium, sodium and potassium. Rubidium colours a flame yellowish violet. Rubidium is used as a 'getter' in vacuum tubes, and as the photoemissive surface in some photocells, and in special glasses. Rubidium is taken up by plants and remains in the ash when burnt. Although present in the human body at a higher concentration (1 part in 105) than many essential elements, it has no known purpose. In 1995 a 'gaseous' cluster of 1000 atoms of rubidium were cooled in a magnetic trap by laser cooling them to a temperature of a few hundred nanoKelvin and forming a new state of matter. The de-Broglie wavelength (arising from matter waves) of the slowly moving atoms spreads out to encompass the cluster, at which point all the atoms behave as one, and a Bose-Einstein condensate of atoms is formed, where all atoms have identical non-zero ground state energies. The rubidium atoms weakly repel each other maintaining the 'gaseous' state. Previously, this Bosonic state has been observed only in the liquid phase. See Helium (superfluid). The ionic crystal RbAg4I5 has the highest room temperature electrical conductivity of any known ionic crystal, about the same as dilute sulphuric acid, and may be of use in thin film batteries. Being of the same group, rubidium is often found in association with potassium, but more so with lithium. Lepidolite, a phyllosilicate of the mica group, K(Li,Al)3(Si,Al)4O10(F,OH)2, may contain up to 3% of rubidium, and 0.04% RbCl in colourless or orangish carnallite, hydrated potassium magnesium chloride, KMgCl3·6H2O. Rubidium is also found in leucite, potassium aluminium silicate, KAlSi2O6; and in zinnwaldite. It also occurs in tea, coffee and cocoa. Rubidium is feebly radioactive, because 28% of all natural rubidium exists as the beta decaying isotope rubidium-87, which has an enormously long halflife of 48,000 Million years. The radioactive decay of rubidium-87 into the stable strontium-87 is used to measure geological ages in rocks (The relative abundance of the two isotopes gives a 'strontium age'). There is only one stable isotope, rubidium-85 at 72% isotopic abundance. 26 radioactive isotopes are known, ranging from the positron emitting rubidium-74 to the electron emitting rubidium-102. Claim to fame: Rubidium has the lowest photoelectric work function (2.1eV) of any element.
|
![]()