
|
36 KRYPTON Kr (Greek: Kryptos = hidden)
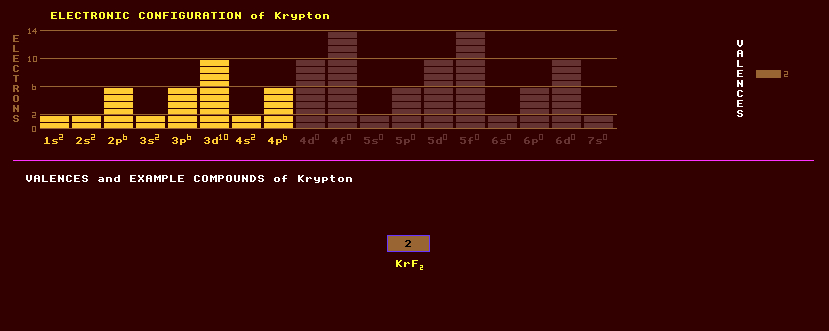
A colourless, odourless noble monatomic gas once thought to be completely inert due to its completed-octet electron shell, but now known to form some compounds. It is present in air at a low 1 part per million by volume, and is obtained by the liquefaction and subsequent fractional distillation of air to separate it from other gases. It is characterised by brilliant green and orange spectral lines, some of which are so sharp that it is used as the fundamental standard of length: 1 metre equalling 1,650,763.73 wavelengths (in a vacuum) of an orange-red line of Krypton-86. This replaces the platinum-iridium alloy bar of one metre length in Paris. Solid krypton is a white crystalline substance with a face-centred cubic structure which is common to all the noble gases. Krypton is used as an inert gas filling in small incandescent filament torch bulbs to increase the luminous efficiency, and as a low-pressure filling for fluorescent lights, and in certain photographic gas discharge lamps for high speed photography. Uses are limited by its high cost. Krypton difluoride, KrF2, a higher fluoride, and a salt of an oxyacid have been reported. Krypton fluoride gas lasers produce ultraviolet light of 0.248nm wavelength, useful in photolithography for silicon chip mask production. Molecular ions of (ArKr)+, and (KrH)+ have been identified and investigated, and evidence is provided for the formation of KrXe or (KrXe)+. Krypton clathrates, where the krypton is physically caged within a larger structure, such as Kr8(H2O)46, and Kr(quinol)2 have been reported. The isotope krypton-85 has found recent application in chemical analysis: kryptonates are formed by imbedding the isotope in various solids, and these are sensitive to chemical reactions at the surface, from which estimates of the concentration of reactants are possible. Naturally occurring krypton is a mixture of six stable isotopes, comprised mostly of krypton-84 (57%), with smaller about-equal amounts of krypton-82, krypton-83 and krypton-86 (12-17%), followed by 2% krypton-80 and 0.3% krypton-78. A further 21 radioactive isotopes of krypton are known, ranging from krypton-71 to krypton-97. Krypton-85, a beta emitter with a halflife of 10.7 years, is one of the many products of the fission of uranium and is released into the atmosphere from nuclear power stations and during re-processing. Its dilution in the atmosphere renders it relatively harmless. Claim to fame:
|
![]()