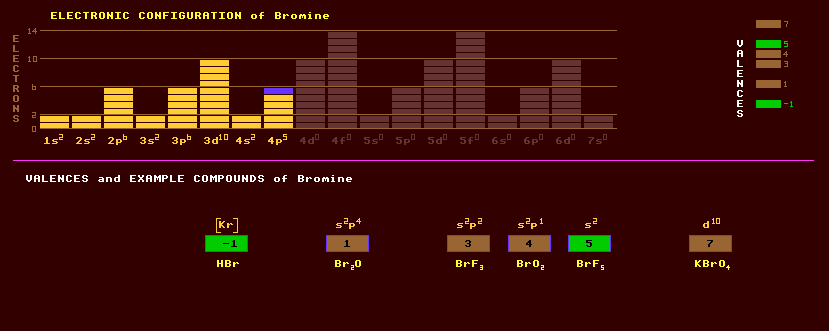

|
35 BROMINE Br (Greek: bromos - smell)
Bromine is a reddish brown, dense, liquid with an obnoxious smell belonging to group 17, the halogens, along with fluorine, chlorine, itself, iodine and astatine. Bromine gives off a highly poisonous red vapour. Bromine is extracted chiefly from sea water or lake evaporites by treating the 'bittern' with chlorine. Bromine is used in halogen-quenched Geiger tubes. Bromine unites with many elements, has a bleaching action like chlorine, and produces painful sores if spilled on the skin. Salts of hydrobromic acid, HBr, are called bromides, which are very soluble in water. Silver bromide, AgBr, is used as a light sensitive crystal in photographic printing paper, and is dissolved by hypo (sodium thiosulphate, Na2S2O3). Bromic acid is HBrO3, a powerful oxidising agent. Salts of perbromic acid, HBrO4, are powerful oxidisers. Bromochlorodifluoromethane, CBrClF2, or BCF, a liquid, is used in fire extinguishers because of its low toxicity. The vapour is 5.7 times denser than air. Another Halon used in fire extinguishers is CF3Br which like CFC's damage the ozone layer. Bromine has a greater potential than chlorine for damaging the ozone layer. Bromoform, CHBr3, is a dense colourless liquid used to separate various minerals by floatation (if the density of the mineral is less than 2.9 then it will float). Ethylene dibromide, BrCH2CH2Br, is added to petrol as a lead scavenger. The lead is produced from decomposition of tetraethyl lead which is also added to petrol as as an anti-knock agent. ('She swallowed a bird to catch a fly' comes to mind, but I don't know why she swallowed a fly). Bromine compounds are used as fumigants, water purifying compounds, dyes, medicines, sanitisers, etc. Bromides have been used as sedatives during the war (bromide tea) and as anti-convulsants in the treatment of epilepsy. Traces of bromine compunds are detectable in the atmosphere, especially the gas methyl bromide, CH3Br, which is produced by ocean organisms. Tyrian purple is a natural purple dye known to the Romans found in certain snails. Bromine exists as two stable isotopes, 51% bromine-79 and 49% bromine-81. A further 23 radioactive isotopes are known, ranging from the positron emitting Br-78 to the electron emitting Br-94. Claim to fame: Bromine has the highest specific heat (76J/ºK/mol) of any element and is the only liquid non-metallic element.
|
![]()