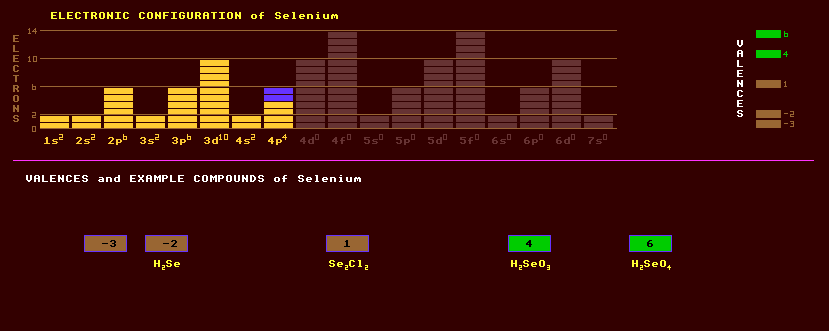

|
34 SELENIUM Se (Greek: Selene - moon)
Selenium is in group 16 along with oxygen, sulphur, itself, tellurium (with which it more closely resembles chemically), and polonium. Selenium is allotropic and assumes various forms: an amorphous selenium is red in powder form or black in vitreous form, and melts at 180 Celsius; a deep red crystalline monoclinic selenium; and a grey crystalline hexagonal (metallic) selenium is produced when other forms are heated above 200 Celsius is an electrical conductor when illuminated and melts at 217 Celsius, this is the most stable form. They are both based on different arrangements of rings of Se8 molecules. Two other allotropic forms are known, both crystallizing in the cubic structure. Selenium has in the past been used as a rectifier when bonded to iron (selenium rectifier). A selenium cell can be of the photoconductive variety where it reduces its resistance in response to light as used in some photographic lightmeters, or the photovoltaic variety where a voltage is induced when light falls on a junction with other materials as used in some solar cells. Like cadmium sulphide, CdS, and cadmium telluride, CdTe, cadmium selenide, CdSe, is used as a II/VI photoconductive semiconductors in the detection of light. A light sensitive photoconductive selenium drum was used in early photocopiers as the printing device. Selenium toner is used to tone black and white photographs an old-looking brown. Selenium is used as a decolouriser for glass, and in selenium glass to colour glass red/orange for use as a filter in colour cinematography, and in red enamels. Selenium forms halides with the halogens like selenium tetrafluoride, SeF4 and selenium hexafluoride, the highest halide of selenium. No iodides of selenium are known. Selenium is found in the flue dusts from the processing of copper sulphide ores are used and from the anode slimes of electrolytic copper refineries. It is also found as the selenides of many heavy metals and in the rare minerals crooksite and clausthalite. Elemental selenium is an essential trace element in humans but toxic above 5mg, however hydrogen selenide and other selenium compounds are extremely toxic and resemble arsenic poisoning in physiological symptoms. Selenium occurs in some soils in such amounts as to make some plants such as locoweed toxic for animals to graze upon. Selenium has six stable isotopes, comprising 1% Se-74, 9% Se-76, 8% Se-77, 24% Se-78, 50% Se-80, and the slightly radioactive selenium-82 at 9%, which is subject to double beta decay and as such has an enormously long half life of 3x1012 years. Altogether, seventeen other radioactive isotopes are known ranging from the inverse beta decaying selenium-68 to the beta decaying selenium-91. Claim to fame: Selenium has the highest photoelectric work function (5.9eV) of any element.
|
![]()