
|
33 ARSENIC As (Greek: arsenikon = yellow orpiment)
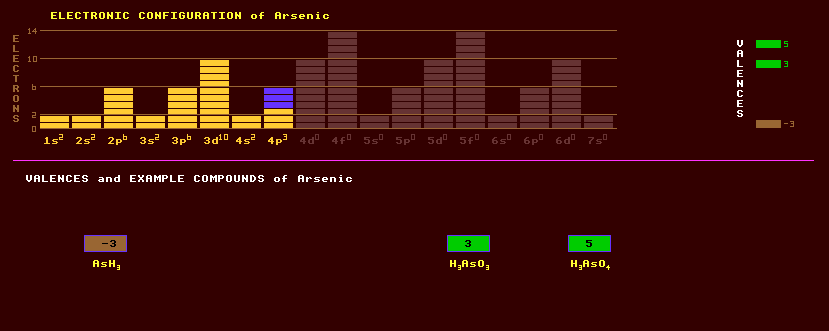
Arsenic is a metalloid in the same group (15) as nitrogen, phosphorus, itself, antimony and bismuth. Arsenic occurs as an impurity in many refined metals. Arsenic can exist in many allotropic forms: The stable form is gamma-arsenic or grey arsenic, which is metallic, soft and brittle, and when heated, does not melt, but sublimes at 615 Celsius, and only under a pressure of 36 atmospheres can it be made to boil (at 814 Celsius). Alpha arsenic or yellow arsenic is formed by the rapid condensation and Beta arsenic or black by the slow condensation of arsenic vapour in an inert atmosphere. Arsenic is added to lead to make lead shot; arsenical copper contains 0.6% arsenic to increase its hardness and strength. Arsenic is also used as an electron donor impurity in germanium semiconductors and semiconducting gallium arsenides used to make LEDs. Arsenic exhibits two valences, 3 and 5. White arsenic (As4O6, arsenic trioxide) is obtained by roasting arsenical ores and is highly poisonous, used as a herbicide and rodenticide and for centuries as a poison; it forms arsenious acid H3AsO3 when dissolved in water, its salts are called arsenites. This oxide is given off as white fumes with a strong smell of garlic when arsenic is heated in air. Arsenic compounds are highly poisonous. The antidote for arsenic (and mercury) poisoning is BAL, British anti-lewisite, dithioglycerol, also effective against lewisite, a WW II poison 'gas'. Arsenic acid, H3AsO4, is formed by the reaction of hot dilute nitric acid with arsenic, or by adding water to arsenic (V) oxide, As4O10, its salts are called arsenates. The fluorides are AsF3 and AsF5. Calcium arsenate and lead arsenate are used as agricultural insecticides. Organo-arsenic compounds are used in wood preservatives and pesticides. Some arsenic containing drugs are still used in medicine, but salvarsen has been superceded by penicillin. Arsine, AsH3, a very poisonous smelly gas, is used in the growing of gallium arsenide crystals for use as a semiconductor. Marsh's test for arsenic makes use of the formation of arsine and its ready decomposition. Trimethyl arsine, As(CH3)3, a very poisonous gas smelling of garlic given off by the mould on Napoleons wallpaper by the decomposition of a green pigment, copper arsenite or Scheele's green, in a damp atmosphere, may have been responsible for his death. Trimethyl arsine is released into the atmosphere by some micro-oganisms. Dimethyl arsine or cacodyl, As2(CH3)4, is a colourless liquid with a hideous smell. Arsenic seems to be beneficial in traces, helping growth. The toxic dose of arsenic trioxide, As2O3, well known as a deliberate poison, is 200mg. Acute poisoning by arsenic causes severe gastro-enteritis, lower levels over prolonged periods hair loss and skin lesions. Arsenic occurs as the bright red-orange translucent crystals of the mineral realgar, As4S4, which gradually crumbles to dust when exposed to light; and as the golden yellow translucent crystals of orpiment, As2S3, once used as a yellow pigment and produced artificially under the name of 'kings yellow'. Orpiment is used in the tanning of hides to remove hair and is also affected by light, though less so. Arsenopyrite or mispickle, FeAsS, is a silver white opaque crystal and an important ore of arsenic, as is enargite, copper arsenic sulphide, Cu3AsS4. Gersdorffite, NiAsS, occurs in no economic amounts. Lollingite, FeAs2, and glaucodot, (Co,Fe)AsS are ores of arsenic. Arsenolite, arsenic oxide, occurs as a white encrustation. Nickeline, NiAs is an ore of nickel like cobaltite, CoAsS and erythrite, Co3(AsO4)2·8H2O are ores of cobalt. Tennantite, Cu12As4S13, occurs in copper and lead deposits. Arsenic occurs natively in hydrothermal veins associated with native silver and sulphides of silver, nickel or cobalt. Allemontite is a naturally occurring compound of arsenic and antimony and is tin-white in colour. Arsenic exists solely as one stable isotope, arsenic-75. Twenty one radioactive isotopes are known, from positron emitting As-66 to electron emitting As-87. Claim to fame: The only element, which at normal pressures, sublimes, that is, its melting point is identical to its boiling point.
|
![]()