
|
32 GERMANIUM Ge Germany
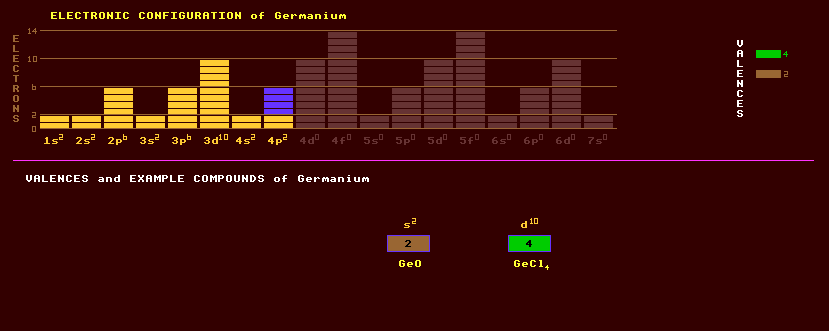
Germanium is a greyish white metalloid belonging to group 14 along with carbon, silicon, tin, and lead and which is used as a semiconductor. It is brittle and crystalline, crystallizing in the cubic system with the same tetrahedral lattice as diamond. Zone refining techniques have lead to the production of ultra high purity germanium (with an impurity of 1 in 1010) for use as a semiconductor. Germanium doped with arsenic or gallium is used as a semiconductor in germanium rectifiers, diodes and transistors, particularly for high currents because of its lower forward voltage drop of only 0.3 volts compared with that of silicon of 0.7 volts. When pressurised to 120kbar, alpha-germanium makes the transition to a high-pressure electrically conducting phase, beta-germanium, which has the same structure as white tin. At higher pressure, a third form, gamma-germanium forms, whose resistance is highly dependant upon pressure, but the resistance/pressure curve exhibits great hysteresis. Germanium is tetravalent and forms a hydride, germane, GeH4, and is also divalent as in GeCl2. The volatile germanium tetrachloride is used in the purification of germanium, the tetrachloride is hydrolysed to germanium dioxide, GeO2, then that is reduced to germanium with hydrogen. Germanium oxide, GeO2, is transparent to infra red radiation and also has a high optical index of refraction making it suitable for use in wide angle camera lenses and microscope objective lenses. Germanium is also transparent to infrared radiation but totally opaque (black) at visible wavelengths, making it suitable for lenses of thermal imaging cameras. Germanium has a very high refractive index of 4.00 at infrared wavelengths. Like gallium, germanium never occurs in concentrated form, and instead is widely but thinly dispersed in many oxide and sulphide minerals. Germanium occurs in coal and in the flue dusts of town-gas gasworks, and it is extracted from the flue dusts of zinc refineries. Germanium also occurs in certain zinc and copper concentrates. Argyrodite, a double sulphide of germanium and silver, is the principle ore of germanium in which it was first discovered. Germanite contains 8% of germanium. Germanium has low concentrations in natural waters because its compounds are very insoluble. Germanium consists of five stable isotopes, 21% of Ge-70, 28% Ge-72, 8% Ge-73, 36% Ge-74 and 7% Ge-76. Seventeen other radioactive isotopes are known ranging from the positron emitting germanium-61 to the electron emitting germanium-84. Claim to fame:
|
![]()