
|
31 GALLIUM Ga (Latin: Gallia = France)
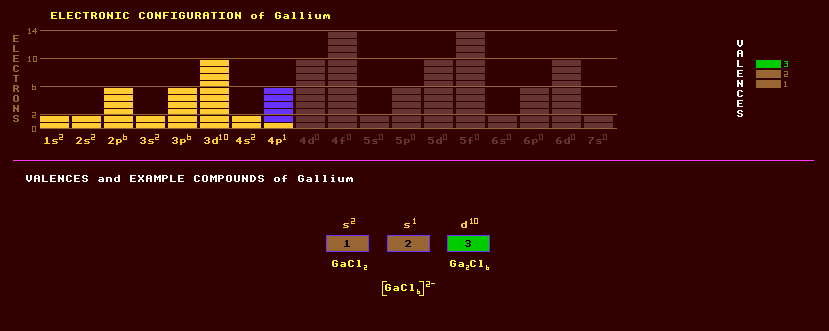
A di- or trivalent metal which has a beautiful silvery appearance when ultra-pure. Gallium has some chemical similarities to aluminium in the same group 13 and to zinc, which is in the adjacent group 12. Gallium is often found as a trace element in diaspore, AlO(OH); sphalerite, (Zn,Fe)S; germanite; bauxite, hydrous aluminium oxides; and coal. Some flue dusts from burning coal contain as much as 1.5% gallium. It is the only metal, except for mercury, caesium, and rubidium which can be liquid near room temperature. It has a melting point of 29.8ºC, and a boiling point of 2204ºC which gives it one of the broadest liquid ranges of any metal making it suitable for use as a high temperature thermometer. Unlike mercury, it has a low vapour pressure even at high temperatures. There is a strong tendency for gallium to remaining liquid below its freezing point, to become a super-cooled liquid. Therefore seeding may be necessary to initiate solidification. The solid metal exhibits a conchoidal fracture similar to glass. Because the metal is one of the few materials that expands on solidifying (by 3.1%) it should not be stored in glass or metal containers, because they may break as the metal solidifies. Gallium wets glass or porcelain and forms a brilliant mirror when painted on glass. Gallium readily alloys with most metals, and has been used as a component of low melting point alloys. Indeed, liquid gallium will readily corrode most metals by diffusing into their crystalline structure. High purity gallium is attacked only slowly by mineral acids. Being trivalent, it can be used to dope semiconductors such as transistors. It has found extensive use in producing solid state light emitting diodes when alloyed with phosphorus, arsenic or aluminium (GaP, GaAs, GaAsP and GaAlAs LEDs) producing light of differing shades of red, orange, yellow and green. It is also used to make infrared laser diodes. Gallium arsenide, which, at infra-red wavelengths, has both a high index of refraction (of 3.48) and a very low absorbtion, has been used to trap light in a never-ending circuit by suspending an amorphous cloud of its crystals in a liquid. The semiconductor gallium nitride, GaN, has a bandgap of 3.45 eV, in comparison with indium nitride, InN, of 1.9eV and aluminium nitride, AlN, 6.2eV. Recently, the long-sought after blue LEDs have been fabricated using indium gallium nitride, being superior to previous and highly inefficient silicon carbide devices which have an indirect energy gap, and zinc selenide which accumulates cracks as it glows. The newer quatenary compound of aluminium, gallium, indium and nitrogen in a 1:1:1:1 ratio, a blue light emitting semiconductor, is uniquely suited as an LED material, for by varying the In/Ga ratio, it will emit light of any colour between yellow and blue and with a hundred fold higher brightness than that of silicon carbide. Magnesium gallate, containing divalent impurities such as Mn+2, is finding use in commercial ultraviolet activated powder phosphors.
Although costing about 15 Million dollars, 30 tons of metallic gallium shielded from cosmic rays at the bottom of a deep mine are used to detect electron neutrinos emitted by all the nuclear reactions in the sun, including those emitted by the pp (proton + proton) fusion reaction to which chlorine-37 neutrino detectors are completely insensitive. The neutrino threshold energy of gallium is just 233keV. (71Ga + Gallium is associated with and widely disseminated in many oxide and sulphide minerals, and although not rare gallium never occurs as a concentrated ore. Gallium is recovered as a by-product of zinc and copper refining. Its toxicity appears to be low. The metal can be supplied in ultra high purity form (99.99999+%) as a consequence of the semiconductor industry. Gallium occurs naturally as two stable isotopes, 60% Ga-69 and 40% Ga-71. Altogether, 22 isotopes of gallium are known, from positron emitting Ga-62 to electron emitting Ga-83. Claim to fame:
|
![]()